61717-82-6
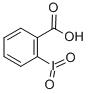
- Product Name:2-Iodoxybenzoic acid
- Molecular Formula:C7H5IO4
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 61717-82-6
Molecular Formula: C7H5IO4
Appearance: White to off-white powder, prills or agglomerates
61717-82-6 Properties
- Molecular Formula:C7H5IO4
- Molecular Weight:280.019
- Appearance/Colour:White to off-white powder, prills or agglomerates
- Vapor Pressure:0mmHg at 25°C
- Melting Point:280 °C
- Refractive Index:1.675
- Boiling Point:331.824°C at 760 mmHg
- Flash Point:154.482°C
- PSA:71.44000
- Density:2.058g/cm3
- LogP:1.75180
61717-82-6 Usage
Chemical Properties
White to off-white powder, prills or agglomerates
Uses
2-Iodoxybenzoic Acid is used in the oxidation of alcohols in organic synthesis. It is also frequently used in the preparation of Dess-Martin periodinane.
Uses
Stabilized 2-Iodoxybenzoic Acid (SIBX)
Definition
ChEBI: A benziodoxole compound having hydroxy and oxo substituents at the 1-position and an oxo substituent at the 3-position.
Synthesis Reference(s)
The Journal of Organic Chemistry, 48, p. 4155, 1983 DOI: 10.1021/jo00170a070
InChI:InChI=1/C7H5IO3/c8-11-6-4-2-1-3-5(6)7(9)10/h1-4H,(H,9,10)
61717-82-6 Relevant articles
The crystal structure of the Dess-Martin periodinane
Schroeckeneder, Albert,Stichnoth, Desiree,Mayer, Peter,Trauner, Dirk
, p. 1523 - 1527,5 (2012)
We report the elusive X-ray structure of the Dess-Martin periodinane (DMP), a hypervalent iodine reagent popular amongst synthetic chemists. In the solid state, the highly crystalline compound forms an intricate coordination polymer held together by intermolecular halogen and hydrogen bonds.
A User-Friendly Entry to 2-Iodoxybenzoic Acid (IBX)
Frigerio, Marco,Santagostino, Marco,Sputore, Simona
, p. 4537 - 4538 (1999)
-
Process for preparing Dess-Martin reagent through solid superacid catalysis
-
Paragraph 0014; 0021-0022; 0024-0025; 0027-0028; 0030-0031, (2021/07/31)
The invention belongs to the technical field of organic synthesis, and discloses a process for preparing a Dess-Martin reagent through solid superacid catalysis. The process comprises the following steps of: S1, oxidation reaction, specifically, mixing potassium bromate and tap water, adding solid superacid HERD-Cat-1, stirring and heating the materials to 40-90 DEG C; adding o-iodobenzoic acid in batches, reacting for 1-8 hours at the temperature after addition of the o-iodobenzoic acid, performing cooling, and then centrifugally washing a product with water and washing with an organic solvent to obtain an IBX wet product; S2, acetylation reaction, specifically, adding the IBX wet product into an organic solvent, performing dissolving and filtering, recovering solid superacid HERD-Cat-1, adding a catalyst and acetic anhydride, heating to 40-80 DEG C, performing a reaction, performing cooling and crystallizing, and performing centrifugation and drying to obtain the Dess-Martin reagent. After the solid superacid is used for catalysis, generation of a large amount of waste acid is avoided, and meanwhile the purity of IBX is high. The process has the advantages of short reaction steps, high chemical purity, simplicity in operation, low cost, high production safety, environmental friendliness and the like, and is suitable for industrial production.
SCALEABLE PREPARATION OF POLYKETIDES
-
Paragraph 0315, (2021/02/12)
Disclosed herein, inter alia, are methods of making polyketide compounds.
Preparation method of β - carotene with high total trans content
-
Paragraph 0047-0049, (2021/09/01)
The invention provides a preparation method of β - carotene with a high total trans content. Starting from the vitamin A derivative, C20 phosphine salt is obtained through reaction with triphenylphosphine, and the C20 phosphine salt is subjected to condensation reaction under the action IBX high iodine compound and base to obtain β - carotene containing cis-trans, and then the β - carotene with high trans content is obtained under the action of a transition metal loading catalyst. The method avoids the use of a strong oxidizing agent in the traditional process, is environmentally friendly and can reduce the peroxidation of β - carotene, so that the reaction yield is increased, and the transition metal catalyst enables the obtained β - carotene content to be full. Trans increase.
METHOD FOR PRODUCING HYPERVALENT IODINE COMPOUND USING HYPOCHLORITE
-
Paragraph 0033-0035, (2020/10/03)
PROBLEM TO BE SOLVED: To provide a method for producing a hypervalent iodine compound as a 2-iodobenzoic acid derivative that is safer and more convenient than the conventional method. SOLUTION: The method includes mixing 2-iodobenzoic acid as a starting material with hypochlorite or hypochlorite aqueous solution. During the reaction, depending on whether acetic acid or acetic anhydride is present or not in the mixture, IBA, IBA-OAc, IBX can be produced separately and selectively. SELECTED DRAWING: None COPYRIGHT: (C)2020,JPO&INPIT
61717-82-6 Process route
-
![1-Benzyloxy-1-oxo-1H-1λ<sup>5</sup>-benzo[d][1,2]iodoxol-3-one](/upload/2023/1/325ddc51-8ed2-490f-9d5b-295ba640d3c2.png)
- 185566-87-4
1-Benzyloxy-1-oxo-1H-1λ5-benzo[d][1,2]iodoxol-3-one

-

- 100-51-6,185532-71-2
benzyl alcohol

-
![1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione](/upload/2023/1/2542863e-52f8-4d84-a5e2-bc4eb8d77d30.png)
- 61717-82-6
1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione
| Conditions | Yield |
|---|---|
|
With water; In dimethylsulfoxide-d6; Equilibrium constant;
|
-

- 88-67-5
2-Iodobenzoic acid

-
![1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione](/upload/2023/1/2542863e-52f8-4d84-a5e2-bc4eb8d77d30.png)
- 61717-82-6
1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione
| Conditions | Yield |
|---|---|
|
With potassium hydrogensulfate; In sulfolane; water; at 50 - 95 ℃; for 6h; Reagent/catalyst; Solvent; Temperature;
|
95.04% |
|
With potassium peroxymonosulfate; In water; at 73 ℃; for 3h;
|
94% |
|
With potassium bromate; at 65 ℃; for 5h; Temperature;
|
94.1% |
|
With potassium bromate; sulfuric acid; at 65 ℃; for 3.6h;
|
93% |
|
With potassium bromate; sulfuric acid; at 68 ℃; for 3.6h;
|
93% |
|
With Oxone; In water; at 70 ℃; for 3h;
|
93% |
|
With dipotassium peroxodisulfate; In sulfolane; water; at 50 - 95 ℃; for 6h;
|
93.12% |
|
With dipotassium peroxodisulfate; sulfuric acid; In water; at 50 - 70 ℃; for 6h; Temperature; Reagent/catalyst; Large scale;
|
92.2% |
|
With Oxone; In water; at 70 ℃; for 3h;
|
89% |
|
With oxone; In water; at 0 - 70 ℃; for 4.5h;
|
89% |
|
With potassium peroxymonosulfate; In water; at 0 - 70 ℃; for 4.83333h;
|
87% |
|
With potassium peroxymonosulfate; In water; at 80 ℃; for 4h;
|
87% |
|
With Oxone; In water; at 70 ℃; for 3h;
|
86% |
|
With Oxone; In water; at 73 ℃; for 3h;
|
84% |
|
With Oxone; In water; at 70 - 75 ℃; for 4h;
|
82% |
|
With oxone; In water; at 105 ℃; for 5h;
|
80% |
|
With oxone; In water; at 72 ℃; for 3h;
|
76% |
|
With Oxone; In water; at 70 ℃; for 3h;
|
74% |
|
With potassium bromate; sulfuric acid; at 55 - 68 ℃; for 4.5h;
|
70% |
|
With Oxone; In water; at 70 ℃; for 3h;
|
63% |
|
With sodium hypochlorite pentahydrate; acetic anhydride; In water; at 20 ℃; for 12h; Solvent;
|
56% |
|
With potassium bromate;
|
|
|
With Oxone; In water; at 70 - 73 ℃; for 3h; Yield given;
|
|
|
With Oxone; In water; at 5 - 70 ℃; for 5h;
|
|
|
With Oxone;
|
|
|
With oxone||potassium monopersulfate triple salt;
|
|
|
With Oxone; In water; at 5 - 70 ℃; for 5h;
|
|
|
With Oxone;
|
|
|
With Oxone;
|
|
|
With potassium bromate; sulfuric acid; at 65 ℃; for 2.5h;
|
|
|
With oxone;
|
|
|
With Oxone; water; at 75 ℃; for 4h;
|
|
|
With potassium bromate; sulfuric acid; at 70 - 85 ℃; for 5.33h;
|
19.7 g |
61717-82-6 Upstream products
-
88-67-5

2-Iodobenzoic acid
-
185566-81-8

1-Methoxy-1-oxo-1H-1λ5-benzo[d][1,2]iodoxol-3-one
-
185566-82-9

1-Ethoxy-1-oxo-1H-1λ5-benzo[d][1,2]iodoxol-3-one
-
185566-83-0

isopropyl 2-iodoxybenzoate
61717-82-6 Downstream products
-
87413-09-0

Dess-Martin periodane
-
135394-69-3

Tetra-n-butylammonium o-iodoxybenzoate
-
131-62-4

1-hydroxy-1,2-benzodioxol-3-(1H)-one
-
185566-87-4

1-Benzyloxy-1-oxo-1H-1λ5-benzo[d][1,2]iodoxol-3-one
Relevant Products
-
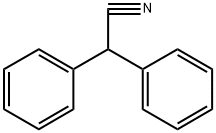
Benzeneacetonitrile, alpha-phenyl-
CAS:86-29-3
-
Sodium hypophosphite monohydrate
CAS:10039-56-2