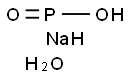
10039-56-2
- Product Name:Sodium hypophosphite monohydrate
- Molecular Formula:NaH2PO2.H2O
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 10039-56-2
Molecular Formula: NaH2PO2.H2O
Appearance: white crystals
10039-56-2 Properties
- Molecular Formula:NaH2PO2.H2O
- Molecular Weight:87.9782
- Appearance/Colour:white crystals
- Melting Point:90 °C
- PSA:59.17000
- Density:0.8 g/cm3 (20oC)
- LogP:0.02390
10039-56-2 Usage
Chemical Properties
White crystals
Uses
Sodium hypophosphite monohydrate is used for electroless nickel plating. Sodium Hypophosphite is capable of reducing nickel ions in solution to metallic nickel on metal substrates as well as on plastic substrates
Uses
Sodium hypophosphite monohydrate is an emulsifier or stabilizer that is a white, odorless, deliquescent granular powder with a saline taste. It is also prepared as colorless, pearly crystalline plates.
Uses
Sodium hypophosphite monohydrate is used for electroless nickel plating agent and water treatment agent. It is also used in petroleum fields. Further, it is used as a food additive. In addition, it reduces nickel ions in solution to metallic nickel on metal substrates as well as on plastic substrates.
Purification Methods
Dissolve it in boiling EtOH, cool and add dry Et2O till all the salt separates. Collect and dry it in vacuum. It is soluble in 1 part of H2O. It liberates PH3 on heating and can ignite spontaneously when heated. The anhydrous salt is soluble in ethylene glycol (33% w/w) and propylene glycol (9.7%) at 25o.
InChI:InChI=1/Na.H3O2P.H2O/c;1-3-2;/h;3H2,(H,1,2);1H2/q+1;;/p-1
10039-56-2 Relevant articles
Phosphaketenes as building blocks for the synthesis of triphospha heterocycles
Heift, Dominikus,Ben?, Zoltán,Grützmacher, Hansj?rg
, p. 11326 - 11330 (2014)
Unsaturated phosphorus compounds, such as phosphaalkenes and phosphaalkynes, show a versatile reactivity in cycloadditions. Although phosphaketenes (R-P=C=O) have been known for three decades, their chemistry has remained limited. Herein, we show that heteroatom-substituted phosphaketenes, R3E-P=C=O (E=Si, Sn), are building blocks for silyl- and stannyl-substituted five-membered heterocycles containing three phosphorous atoms. The structure of the heterocyclic anion depends on the nature of the tetrel atom involved. Although the silyl analogue [P3C 2(OSiR3)2]- is an aromatic 1,2,4-triphospholide, the stannyl compound [P(CO)2(PSnR 3)2]- is a 1,2,4-triphosphacyclopenta-3,5- dionate with a delocalized OCPCO fragment. Because of their anionic character, these compounds can easily be used as building blocks, for example, in the preparation of a silyl-functionalized hexaphosphaferrocene or the parent 1,2,4-triphosphacyclopenta-3,5-dionate [P(CO)2(PH)2] -. NMR spectroscopic investigations and computations have shown that the heterocycle-formation reactions presented herein are remarkably complex. Cycle time! The reaction of Na(OCP) with tetrelhalides, R3EX (E=Si, Sn), gives silyl- and stannyl-substituted five-membered heterocycles containing three phosphorous atoms. Phosphaketenes, R3E-P=C=O, are intermediates, which react with Na(OCP) as a P- transfer reagent to the final products (see scheme; Tf=triflucromethanesulfonyl).
Synthesis of H-Phosphonate Intermediates and Their Use in Preparing the Herbicide Glyphosate
-
Paragraph 0044; 0036, (2014/10/16)
The esterfication of hypophosphorous acid is followed by reaction with another molecule of alcohol under the action of a nickel catalyst to provide a green method for the preparation of H-phosphonate diesters. This method avoids the need for any stoichiometric chlorine unlike those based on phosphorous trichloride.
10039-56-2 Process route
-

- 7758-19-2
sodium chlorite

-

-
phosphorous

-

- 10039-56-2
sodium hypophosphite

-

-
sodium phosphite

-

-
sodium subphosphate

-

-
sodium phosphate
| Conditions | Yield |
|---|---|
|
In water;
|
-

- 12058-85-4
sodium phosphide

-

- 7732-18-5
water

-

- 10039-56-2
sodium hypophosphite

-

-
sodium phosphite

-

- 1333-74-0
hydrogen

-

- 7803-51-2
phosphan

-

- 1310-73-2
sodium hydroxide
| Conditions | Yield |
|---|---|
|
In water; reaction of Na3P with H2O;;
|
10039-56-2 Upstream products
-
7758-19-2

sodium chlorite
-
12058-85-4

sodium phosphide
-
7732-18-5

water
-
1310-73-2

sodium hydroxide
10039-56-2 Downstream products
-
86552-32-1

(4-phenylbutyl)phosphinic acid
-
7440-02-0

nickel
-
13598-36-2

phosphonic Acid
-
86119-84-8

phosphoric acid
Relevant Products
-
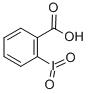
2-Iodoxybenzoic acid
CAS:61717-82-6
-
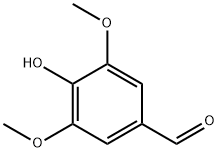
3,5-Dimethoxy-4-hydroxybenzaldehyde
CAS:134-96-3