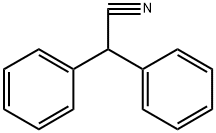
86-29-3
- Product Name:Benzeneacetonitrile, alpha-phenyl-
- Molecular Formula:C14H11N
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 86-29-3
Molecular Formula: C14H11N
Appearance: white to creamy or faint yellow crystalline powder
86-29-3 Properties
- Molecular Formula:C14H11N
- Molecular Weight:193.248
- Appearance/Colour:white to creamy or faint yellow crystalline powder
- Vapor Pressure:0.000282mmHg at 25°C
- Melting Point:71-73 °C(lit.)
- Refractive Index:1.584
- Boiling Point:322.3 °C at 760 mmHg
- Flash Point:151.6 °C
- PSA:23.79000
- Density:1.076 g/cm3
- LogP:3.34208
86-29-3 Usage
Chemical Properties
Diphenylacetonitrile is a white to creamy or faint yellow crystalline powder, Soluble in ethanol, ether. It is obtained by bromination and condensation of phenylacetonitrile.Diphenylacetonitrile is mainly used as an intermediate to manufacture API which deals in treatment of respiratory stimulant. It is used to manufacture APIs like stomach amine, Aminepentamide Sulphate, Diphenoxylate, Diphenylacetaldehyde, Doxapram, Loperamide, Methadone.
Uses
Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceuticals.Diphenylacetonitrile is used to synthesize isocyanate, which is further prepared into UV paint, PU paint, transparent elastomer and adhesive, etc. In addition, it is also used in polyamide and epoxy resin industries.
Preparation
Diphenylacetonitrile can be prepared in good yield by the dehydration of the amide of diphenyl acetic acid with phosphorous oxychloride. Diphenylacetonitrile undergoes anhydrous condensation with ethyl-4-bromo-butyrate. This is followed by its hydrolysis in alkaline medium that is used in the quantitative determination of 3-cyano-3,3-diphenylpropionic acid.
Application
Diphenylacetonitrile undergoes anhydrous condensation with ethyl-4-bromo-butyrate. This is followed by its hydrolysis in alkaline medium that is used in the quantitative determination of 3-cyano-3,3-diphenylpropionic acid.
Reactions
Methadone is synthesised by alkylation of diphenylacetonitrile using 2-dimethylaminopropylchloride in the presence of sodamide.Dextromoramide is synthesised by alkylation of diphenylacetonitrile using 1-morpholinyl-2- chloropropane in the presence of sodamide.Diphenylacetonitrile in the presence of sodium amide is alkylated with 1-ethyl-3-chlorpyrrolidine, giving (1-ethyl-3-pyrrolidinyl) diphenylacetonitrile.Isopropamide, is synthesized by alkylating diphenylacetonitrile with di-isopropylaminoethylchloride in the presence of sodium amide, and the subsequent hydrolysis of the nitrile group of the resulting compound to an amide group.
Synthesis Reference(s)
The Journal of Organic Chemistry, 35, p. 3253, 1970 DOI: 10.1021/jo00835a016
General Description
Diphenylacetonitrile can be prepared in good yield by the dehydration of the amide of diphenyl acetic acid with phosphorous oxychloride.
Safety Profile
Poison by ingestion, intraperitoneal, and intravenous routes. Moderately toxic by subcutaneous route. Questionable carcinogen with experimental carcinogenic and tumorigenic data. When heated to decomposition it emits toxic fumes of NOx, and CN-. See also NITRILES.
Synthesis
Add the 250mL ethyl acetate in 500mL glass there-necked flask, open and stir, add the 150g phenylcarbinol, add sodium methylate 80g, 70 ℃ of stirring reactions 2 hours are down to room temperature, add benzyl cyanide 120ml.Be heated to 110 ℃, distillation reaction 10h.Reaction naturally cools to room temperature after finishing, and adds the extraction of 200ml ethyl acetate and 200ml water, tells lower aqueous layer.The upper strata ethyl acetate layer adds water, and washing once adds anhydrous sodium sulfate drying.Concentrating under reduced pressure is removed ethyl acetate.Then in oily matter, add a small amount of dehydrated alcohol crystallisation by cooling, the crystallized product oven dry.Yield 90%, purity 99.0% (GC).
Purification Methods
Crystallise the nitrile from EtOH or pet ether (b 90-100o). [Beilstein 9 H 674, 9 IV 2505.]
InChI:InChI=1/C14H11N/c15-11-14(12-7-3-1-4-8-12)13-9-5-2-6-10-13/h1-10,14H
86-29-3 Relevant articles
-
Ginsburg,Baizer
, p. 2254 (1949)
-
Preparation of unsaturated nitriles by the modification of Mitsunobu-Wilk procedure
Aesa,Baan,Novak,Szantay
, p. 1545 - 1550 (1995)
An efficient one-step procedure for the conversion of unsaturated alcohols into the corresponding nitriles by the modification of Mitsunobu-Wilk reaction is described.
Radical trifunctionalization of hexenenitrile via remote cyano migration
Chang, Chenyang,Wu, Xinxin,Zhang, Huihui,Zhu, Chen
supporting information, p. 1005 - 1008 (2022/02/01)
A novel radical-mediated trifunctionalization of hexenenitriles via the strategy of remote functional group migration is disclosed. A portfolio of functionalized hexenenitriles are employed as substrates. After difunctionalization of the unactivated alken
Rapid and Simple Access to α-(Hetero)arylacetonitriles from Gem-Difluoroalkenes
Hu, Dandan,Liu, Jiayue,Ren, Hongjun,Song, Jinyu,Zhang, Jun-Qi,Zhu, Guorong
supporting information, p. 786 - 790 (2022/01/28)
A scalable cyanation of gem-difluoroalkenes to (hetero)arylacetonitrile derivatives was developed. This strategy features mild reaction conditions, excellent yields, wide substrate scope, and broad functional group tolerance. Significantly, in this reacti
Synthesis of Succinonitrile Derivatives by Homocoupling from Cyanohydrin Derivatives with a Low-Valent Titanium Reagent
Endo, Ryusei,Kishida, Atsushi,Matsunaga, Kazuma,Nagasawa, Kokoro,Takatori, Kazuhiko
, (2022/02/07)
A method is described for synthesizing succinonitrile derivatives bearing alkyl or aryl substituents from cyanohydrin derivatives using low-valent titanium. The active species in this reaction is proposed to be a resonance hybrid of the TiIV nitrile enolate and TiIII alkyl radical.
HCl·DMPU-assisted one-pot and metal-free conversion of aldehydes to nitriles
Hammond, Gerald B.,Mudshinge, Sagar R.,Potnis, Chinmay S.,Xu, Bo
supporting information, p. 4161 - 4164 (2020/07/14)
We report an efficient HCl·DMPU assisted one-pot conversion of aldehydes into nitriles. The use of HCl·DMPU as both an acidic source as well as a non-nucleophilic base constitutes an environmentally mild alternative for the preparation of nitriles. Our protocol proceeds smoothly without the use of toxic reagents and metal catalysts. Diverse functionalized aromatic, aliphatic and allylic aldehydes incorporating various functional groups were successfully converted to nitriles in excellent to quantitative yields. This protocol is characterized by a broad substrate scope, mild reaction conditions, and high scalability. This journal is
86-29-3 Process route
-

- 198337-87-0
C20H16FNO

-

- 119-61-9
benzophenone

-

- 4695-13-0
2,2-diphenylacetamide

-

- 86-29-3
Diphenylacetonitrile

-

- 5267-35-6
N-(diphenylmethyl)acetamide

-

- 22800-71-1
N-(diphenylmethylene)acetamide

-

- 1375263-32-3
1-acetyl-3,3-diphenylaziridin-2-one

-

- 371-40-4
4-fluoroaniline
| Conditions | Yield |
|---|---|
|
With lithium perchlorate; In water; acetonitrile; Electrolysis;
|
-

- 88284-48-4
2-(trimethylsilyl)phenyl trifluoromethanesulfonate

-

- 109-77-3
malononitrile

-

- 86-29-3
Diphenylacetonitrile

-

- 3759-28-2
(2-cyanophenyl)acetonitrile

-

- 92427-55-9
2,2-diphenylmalononitrile

-

- 127667-03-2
2-(cyano(phenyl)methyl)benzonitrile
| Conditions | Yield |
|---|---|
|
With potassium fluoride; 18-crown-6 ether; In tetrahydrofuran; at 20 ℃; for 4h;
|
57% 7% 1% 3% |
86-29-3 Upstream products
-
64-17-5

ethanol
-
43002-68-2

2,2-diphenyl-acetimidic acid ethyl ester; hydrochloride
-
4695-13-0

2,2-diphenylacetamide
-
3122-21-2

2,2,3,3-tetraphenylsuccinonitrile
86-29-3 Downstream products
-
34604-62-1

2,2-diphenyl-3-pyrrolidin-1-yl-propionitrile
-
858197-74-7

3-morpholin-4-yl-2,2-diphenyl-propionitrile
-
59289-98-4

2,2-diphenyl-4-pyrrolidino-butyronitrile
-
112972-12-0

2,2-diphenyl-5-pyrrolidino-valeronitrile; hydrochloride
Relevant Products
-
beta-Diphosphopyridine nucleotide
CAS:53-84-9
-
2-Iodoxybenzoic acid
CAS:61717-82-6