163041-94-9
- Product Name:E3,Z8,Z11-Tetradecatriene acetate
- Molecular Formula:C16H26O2
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 163041-94-9
Molecular Formula: C16H26O2
163041-94-9 Properties
- Molecular Formula:C16H26O2
- Molecular Weight:250.3764
- Boiling Point:333.6±31.0 °C(Predicted)
- PSA:26.30000
- Density:0.903±0.06 g/cm3(Predicted)
- LogP:4.92620
163041-94-9 Usage
Uses
(3E,8Z,11Z)-Tetradeca-3,8,11-trienyl Acetate is a compound found in the pheromones. It is a major sex pheromone component of the tomato pest.
InChI:InChI=1S/C16H26O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-18-16(2)17/h10-15H,3-9H2,1-2H3
163041-94-9 Relevant articles
Titanium(II)-based Z-reduction of alkynes: Stereo- and regio-specific Z-dideuteriation of conjugated and methylene-skipped ynes
Hungerford, Natasha L.,Kitching, William
, p. 1697 - 1698 (1996)
A TiII-mediated, stereo- and regio-specific reduction of isolated, conjugated and methylene skipped poly-ynes to the corresponding Z-dideuterio polyenes in a one-pot procedure with D2O as deuterium source is described, and this methodology (using H2O) is applied to the synthesis of (3E,8Z,11Z)-tetradeca-3,8,11-trienyl acetate, the major sex attractant of Scrobipalpuloides absoluta, a destructive pest of tomatoes.
A new and efficient synthesis of (3E,8Z,11Z)-tetradeca-3,8,11-trienyl acetate, the major sex pheromone component of the tomato leafminer Tuta absoluta
Cabezas, Jorge A.
, p. 407 - 410 (2019)
An efficient synthesis of (3E,8Z,11Z)-tetradeca-3,8,11-trienyl acetate, the major sex pheromone component of the tomato leafminer moth, Tuta absoluta, was accomplished. The synthesis started with but-3-yn-1-ol and gave an overall yield of 41%.
Microscale, random reduction: Application to the characterization of (3E,8Z,11Z)-3,8,11-tetradecatrienyl acetate, a new lepidopteran sex pheromone
Attygalle, Athula B.,Jham, Gulab N.,Svatos, Ale,Frighetto, Rosa T. S.,Meinwald, Jerrold,Villela, Evaldo F.,Ferrara, Fernando A.,Uchoa-Fernandes, Manoel A.
, p. 5471 - 5474 (1995)
The major sex attractant released by Scrobipalpuloides absoluta, a devastating tomato pest, was identified as (3E,8Z,11Z)-3,8,11-tetradecatrienyl acetate by a novel strategy involving the random reduction of double bonds. The technique is applicable to double bond localization of polyunsaturated compounds available even in nanogram quantities. The triene ester was synthesized by a stereospecific procedure and shown to be highly attractive to conspecific males and identical to the natural substance.
Method for synthesizing master-tomato-moth pheromone
-
Paragraph 0020; 0022; 0029-0030, (2020/01/11)
The invention discloses a synthetic method for female tomato hornworm pheromone. The synthetic method comprises the following steps: adopting (5Z,8Z)-undecadienal and diethyl 3-(tetrahydro-2H-pyran-2-yloxy)propylphosphonate to perform a wittig-hornor reaction s as to generate a compound 3 by taking butyl lithium as alkali; under the condition of taking p-TsOH-catalyzed methanol as a solvent, performing de-protection to generate a compound 4; and reacting the compound 4 with acetic anhydride to generate a compound a (3E, 8Z, 11Z)-14C-OAc by taking triethylamine as alkaline and taking dichloromethane as a solvent. Or, the synthetic method comprises the following steps: adopting a compound 5 and a compound 6 to perform a wittig-hornor reaction so as to generate the compound 3 by taking butyl lithium as alkali; performing de-protection to generate a chemical substance (3E, 8Z, 11Z)-tetradecatrieneacetate-1-alcohol under the catalysis of p-TsOH by taking the methanol as a solvent; and reacting the (3E, 8Z, 11Z)-tetradecatrieneacetate-1-alcohol with acetic anhydride to generate a chemical substance a by taking dichloromethane as the solvent and taking triethylamine as alkali. According to the synthetic method, high-risk high-pollution LiAlH4 and liquid ammonia are avoided, and the conversion rates of raw materials are improved.
Preparation of stereochemically pure E- and Z-alkenoic acids and their methyl esters from bicyclo[n.1.0]alkan-1-ols. Application in the synthesis of insect pheromones
Zubrytski,Kananovich,Matiushenkov
, p. 813 - 823 (2017/08/02)
Oxidative cleavage of exo- and endo-alkyl- and hydroxyalkyl-substituted bicyclo[n.1.0]alkan-1-ols with (diacetoxy-λ3-iodanyl)benzene gave the corresponding methyl alkenoates exclusively with E or Z configuration of the double bond. This reaction was used as the key stage in the syntheses of stereoisomerically pure components of pest insect pheromones: (E)-dodec-9-en-1-yl acetate (European pine shoot moth Rhyacionia buoliana), (Z)-tetradec-11-en-1-yl acetate (European oak leafroller Tortrix viridana), and (3E,8Z,11Z)-tetradeca-3,8,11-trien-1-yl acetate (tomato leafminer Tuta absoluta).
An Improved and Convenient New Synthesis of the Pheromone Components of the Tomato Leafminer Tuta absoluta
Puigmartí, Marc,Bosch, Ma Pilar,Guerrero, Angel
, p. 961 - 968 (2015/03/30)
A convenient new synthesis of the two pheromone components of the tomato pest Tuta absoluta in high overall yields and high stereoselectivity (30% yield, E,Z,Z 97% for the major compound and 23% yield, E,Z 99% for the minor component) is reported. The approaches compare favorably with others previously described.
163041-94-9 Process route
-

- 108-24-7
acetic anhydride

-

- 166901-15-1
(E3,Z8,Z11)-3,8,11-tetradecatrienyl alcohol

-

- 163041-94-9
(3E,8Z,11Z)-3,8,11-tetradecatrien-1-yl acetate
| Conditions | Yield |
|---|---|
|
With dmap; In toluene; at 50 - 70 ℃; for 1.5h;
|
100% |
|
With dmap; triethylamine; In tetrahydrofuran; at 0 - 20 ℃; for 4h; Inert atmosphere;
|
98% |
|
With dmap; triethylamine; In tetrahydrofuran; at 20 ℃; for 4h; stereoselective reaction; Inert atmosphere;
|
96% |
|
With triethylamine; In dichloromethane; at 0 - 5 ℃; for 1h;
|
80% |
|
With pyridine; for 1h;
|
67% |
|
In pyridine; Yield given;
|
|
|
With pyridine;
|
|
|
With pyridine; for 1h; Ambient temperature;
|
0.24 g |
-

- 75-36-5
acetyl chloride

-

- 166901-15-1
(E3,Z8,Z11)-3,8,11-tetradecatrienyl alcohol

-

- 163041-94-9
(3E,8Z,11Z)-3,8,11-tetradecatrien-1-yl acetate
| Conditions | Yield |
|---|---|
|
With triethylamine; In diethyl ether;
|
94% |
163041-94-9 Upstream products
-
108-24-7

acetic anhydride
-
166901-15-1

(E3,Z8,Z11)-3,8,11-tetradecatrienyl alcohol
-
62992-46-5

1-(tetrahydropyranyloxy)-4-pentyn
-
6261-21-8

2-pent-2-ynyloxy-tetrahydro-pyran
Relevant Products
-
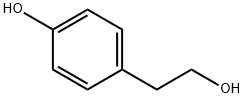
4-Hydroxyphenethyl alcoholCAS NO.: 501-94-0
CAS:501-94-0
-
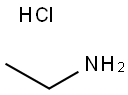
Ethanamine,hydrochloride (1:1)
CAS:557-66-4
-
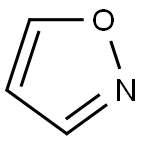
Isoxazole
CAS:288-14-2