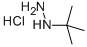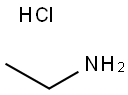
557-66-4
- Product Name:Ethanamine,hydrochloride (1:1)
- Molecular Formula:C2H7N.HCl
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 557-66-4
Molecular Formula: C2H7N.HCl
Appearance: white to off-white crystals
557-66-4 Properties
- Molecular Formula:C2H7N.HCl
- Molecular Weight:81.5452
- Appearance/Colour:white to off-white crystals
- Vapor Pressure:1130mmHg at 25°C
- Melting Point:107-108 °C(lit.)
- Refractive Index:1.4202 (estimate)
- Boiling Point:14.2 °C at 760 mmHg
- PSA:26.02000
- Density:1,22 g/cm3
- LogP:1.46730
557-66-4 Usage
Chemical Properties
white to off-white crystals
Uses
Ethylamine Hydrochloride a chemical used in various chemical organic syntheses and biological studies. It is used in production of resins and rubber latex; also used in oil refining and organic syntheses.
Application
Ethylaminehydrochloride is used as a raw material for organic synthesis. Its free base ethylamine is used in the diethyldiazene, dimethylolethyltriazone, ethylcyanopyrolidone disperse and 1,3-diethylthiourea. It is a precursor used for the preparation of benzilnitrate, detergents, rayon, rocket propellant and alkyl isocyanates, which finds application in the manufacture of pharmaceuticals.
Preparation
Ethylamine can be synthesized by ethanol and ammonia are combined in the presence of an oxide catalyst:CH3CH2OH + NH3 → CH3CH2NH2 + H2OEthylamine could then be solvent extracted or boiled out of hoffman turned to Hydrochloride with HCl and then freebased with caustic to be distilled pure.of course an excess of caustic when freebasing would be a good idea as it would hold onto most of the water created from the basing of a hydrochloride with caustic.
Purification Methods
Crystallise the hydrochloride from absolute EtOH or MeOH/CHCl3, wash with dry ether and dry it in a vacuum. [Beilstein 4 IV 310.]
Precautions
Hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents.
InChI:InChI=1/C2H7N.ClH/c1-2-3;/h2-3H2,1H3;1H
557-66-4 Relevant articles
Green method for catalyzing reduction reaction of aliphatic nitro derivative
-
Paragraph 0005-0006; 0009-0012, (2021/07/31)
The invention relates to a green method for catalyzing reduction reaction of aliphatic nitro derivatives. According to the method, non-transition metal compounds, namely triethyl boron and potassium tert-butoxide, are used as a catalytic system for the first time, an aliphatic nitro derivative and pinacolborane which is low in price and easy to obtain are catalyzed to be subjected to a reduction reaction under mild conditions, and an aliphatic amine hydrochloride product is synthesized after acidification with a hydrochloric acid aqueous solution. Compared with a traditional method, the method generally has the advantages that the catalyst is cheap and easy to obtain, operation is convenient, and reaction is safe. The selective reduction reaction of the aliphatic nitro derivative catalyzed by the non-transition metal catalyst and pinacol borane is realized for the first time, and the aliphatic amine hydrochloride product is synthesized through acidification treatment of the hydrochloric acid aqueous solution, so that a practical new reaction strategy is provided for laboratory preparation or industrial production.
Cobalt-Catalyzed Deoxygenative Hydroboration of Nitro Compounds and Applications to One-Pot Synthesis of Aldimines and Amides
Gudun, Kristina A.,Hayrapetyan, Davit,Khalimon, Andrey Y.,Segizbayev, Medet,Slamova, Ainur,Zakarina, Raikhan
, (2021/11/30)
The commercially available and bench-stable Co(acac)2 ligated with bis[(2-diphenylphosphino)phenyl] ether (dpephos) was employed for selective room temperature hydroboration of nitro compounds with HBPin (TOF up to 4615 h?1), tolerating halide, hydroxy, amino, ether, ester, lactone, amide and heteroaromatic functionalities. These reactions offered a direct access to a variety of N-borylamines RN(H)BPin, which were in situ treated with aldehydes and carboxylic acids to produce a series of aldimines and secondary carboxamides without the need for dehydrating and/or coupling reagents. Combination of these transformations in a sequential one-pot manner allowed for direct and selective synthesis of aldimines and secondary carboxamides from readily available and inexpensive nitro compounds.
Hydrosilylative reduction of primary amides to primary amines catalyzed by a terminal [Ni-OH] complex
Bera, Jitendra K.,Pandey, Pragati
supporting information, p. 9204 - 9207 (2021/09/20)
A terminal [Ni-OH] complex1, supported by triflamide-functionalized NHC ligands, catalyzes the hydrosilylative reduction of a range of primary amides into primary amines in good to excellent yields under base-free conditions with key functional group tolerance. Catalyst1is also effective for the reduction of a variety of tertiary and secondary amides. In contrast to literature reports, the reactivity of1towards amide reduction follows an inverse trend,i.e., 1° amide > 3° amide > 2° amide. The reaction does not follow a usual dehydration pathway.
Transition metal-free catalytic reduction of primary amides using an abnormal NHC based potassium complex: Integrating nucleophilicity with Lewis acidic activation
Bhunia, Mrinal,Sahoo, Sumeet Ranjan,Das, Arpan,Ahmed, Jasimuddin,Sreejyothi,Mandal, Swadhin K.
, p. 1848 - 1854 (2020/03/03)
An abnormal N-heterocyclic carbene (aNHC) based potassium complex was used as a transition metal-free catalyst for reduction of primary amides to corresponding primary amines under ambient conditions. Only 2 mol% loading of the catalyst exhibits a broad substrate scope including aromatic, aliphatic and heterocyclic primary amides with excellent functional group tolerance. This method was applicable for reduction of chiral amides and utilized for the synthesis of pharmaceutically valuable precursors on a gram scale. During mechanistic investigation, several intermediates were isolated and characterized through spectroscopic techniques and one of the catalytic intermediates was characterized through single-crystal XRD. A well-defined catalyst and isolable intermediate along with several stoichiometric experiments, in situ NMR experiments and the DFT study helped us to sketch the mechanistic pathway for this reduction process unravelling the dual role of the catalyst involving nucleophilic activation by aNHC along with Lewis acidic activation by K ions.
557-66-4 Process route
-

-
ethyl-dithiocarbamic acid ; mercury (II)-compound with mercury chloride

-

- 542-85-8
Ethyl isothiocyanate

-

- 557-66-4
ethanamine hydrochloride
| Conditions | Yield |
|---|---|
|
at 160 - 165 ℃;
|
-

- 1113-31-1
dichlorodimethylaminoborane

-

- 75-04-7,85404-22-4
ethylamine

-

-
(BNHC2H5NC2H5)3

-

- 4375-83-1
tris(dimethylamino)borane

-

- 867-97-0
tris(diethylamino)borane

-

- 557-66-4
ethanamine hydrochloride
| Conditions | Yield |
|---|---|
|
In Petroleum ether;
|
|
|
In petroleum ether;
|
557-66-4 Upstream products
-
24948-82-1

N-chloroethylamine
-
41890-10-2

N-ethyl-benzamidine; hydrochloride
-
60-29-7

diethyl ether
-
75-04-7

ethylamine
557-66-4 Downstream products
-
6304-26-3

N-ethyl-N-(2-pyridin-2-ylethyl)amine
-
41891-13-8

ethyl carbamoyl chloride
-
86310-83-0

1-ethyl-4-p-tolyl-piperidin-4-ol
-
5877-76-9

N-ethylhexadecylamine
Relevant Products
-
tert-Butylhydrazine hydrochlorideCAS NO.: 7400-27-3
CAS:7400-27-3
-
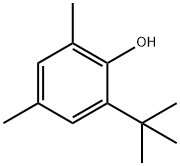
2-(tert-Butyl)-4,6-dimethylphenol
CAS:1879-09-0
-
E3,Z8,Z11-Tetradecatriene acetate
CAS:163041-94-9