1119-94-4
- Product Name:1-Dodecanaminium,N,N,N-trimethyl-, bromide (1:1)
- Molecular Formula:C15H34NBr
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 1119-94-4
Molecular Formula: C15H34NBr
Appearance: white to slightly yellow powder
1119-94-4 Properties
- Molecular Formula:C15H34NBr
- Molecular Weight:308.346
- Appearance/Colour:white to slightly yellow powder
- Melting Point:246 °C
- Refractive Index:1.5260 (estimate)
- Flash Point:246 °C
- PSA:0.00000
- Density:1.1566 (rough estimate)
- LogP:1.61750
1119-94-4 Usage
Chemical Properties
white to slightly yellow powder
Uses
Dodecyltrimethylammonium Bromide is a quaternary ammonium salt useful as paint strippers, foaming stabilizers, and bactericidal lotions.
Uses
(1-Dodecyl)trimethylammonium bromide is used as a surfactant, as an emulsifier of rubber and asphalt and as an anti-static agent of synthetic fiber. It acts as an ionic surfactant and useful in the preparation of gold nanopartilces along with sodium dodecylsulfate. It is also used as paint strippers and foaming stabilizers. It is used to prepare dodecyltrimethylammonium bromide. By using conductimetry, density measurements, and small angle neutron scattering, it is used to study the micelle formation in water-dimethylsulfoxide mixture. Further, it is a useful biochemical for proteomics research.
Uses
Dodecyltrimethylammonium bromide has been used in a study to assess the distribution of binary mixed counterions in surfactant adsorbed films. It has also been used in a study to investigate chain length compatibility of surfactants for extraction of organophosphorous pesticides.
Definition
ChEBI: A quarternary ammonium cation having one dodecyl and three methyl substituents around the central nitrogen.
General Description
Dodecyltrimethylammonium bromide is a quaternary ammonium surfactant.
Flammability and Explosibility
Notclassified
Purification Methods
Purify the salt by repeated crystallisation from acetone. Wash it with diethyl ether and dry it in a vacuum oven at 60o [Dearden & Wooley J Phys Chem 91 2404 1987]. [Beilstein 4 IV 798.]
InChI:InChI=1/C15H34N.BrH/c1-5-6-7-8-9-10-11-12-13-14-15-16(2,3)4;/h5-15H2,1-4H3;1H/q+1;/p-1
1119-94-4 Relevant articles
Properties of Dilute Aqueous Solutions of Double-Chain Surfactants, Alkyldodecyldimethylammonium Bromides with a Change in the Length of the Alkyl Chains
Hiramatsu, Koichi,Kameyama, Keiichi,Ishiguro, Ryo,Mori, Masaki,Hayase, Hisao
, p. 1903 - 1910 (2003)
A series of cationic surfactants, dialkyldimethylammonium bromides with dodecyl as the primary alkyl chain and with methyl, ethyl, propyl, butyl, hexyl, octyl, and decyl as the second alkyl chain, as well as those with symmetric alkyl chains, dioctyl, didecyl and didodecyl ones, were synthesized, and their properties were investigated through measurements of the conductivity and air-liquid surface tension for their aqueous solutions to determine their critical micelle concentrations (cmc) and surface adsorption parameters in the formulation according to a two-dimensional lattice model in the form of the Frumkin equation. The change in cmc revealed that the free energy to transfer from water to the micelle per methylene unit is significantly small for asymmetric double-chain surfactants with a shorter second alkyl chain, and it approaches as elongating the second alkyl to those for the single-chain and symmetric double-chain surfactants. The free energy to transfer to an air-solution interface decreased approximately linearly with the total length of the hydrocarbon chains for all of the species examined. The lattice area for a symmetric double-chain surfactant molecule decreased with the length of its hydrocarbons. In a series of asymmetric ones, it showed a maximum for that with hexyl in its second alkyl.
Micellar effects upon the rate of alkaline hydrolysis of triflusal
Al-Lohedan, Hamad A.,Al-Blewi, Fawziah F.,Rafiquee,Issa, Zuheir A.
, p. 321 - 327 (2015)
The rate of hydrolysis for triflusal was measured at varying concentrations of NaOH at four different temperatures (i.e. 25, 35, 45 and 55 °C). The micelles of cetyltrimethylammonium bromide (CTABr), cetyltrimethylammonium chloride (CTACl), cetyltrimethylammonium hydroxide (CTAOH) and dodecyltrimethyl-ammonium bromide (DTABr) had catalytic effect on the rate of hydrolysis. CTABr, CTACl and DTABr gave maxima like curve for the rate-[surfactant] plot while CTAOH gave plateau like curve. The anionic sodium dodecyl sulfate (SDS) did not influence the rate of alkaline hydrolysis of triflusal. The non-ionic Brij-35 inhibited the rate of the hydrolytic reaction. The catalytic effect by cationic micelles was treated by applying the pseudophase ion exchange model while the inhibitive effect by non-ionic micelles has been described by using the Poisson-Boltzmann pseudophase model. The variation in kψ with the change in [surfactant] was used to determined various kinetic parameters e.g., binding constant (Ks), and micellar rate constant (km). The addition of electrolytes decreased the reaction rate in CTABr and CTAOH micelles.
-
Bunton,C.A. et al.
, p. 7393 - 7400 (1970)
-
Striking improvement in peroxidase activity of cytochrome c by modulating hydrophobicity of surface-functionalized gold nanoparticles within cationic reverse micelles
Maiti, Subhabrata,Das, Krishnendu,Dutta, Sounak,Das, Prasanta Kumar
, p. 15021 - 15030 (2012)
This work demonstrates a remarkable enhancement in the peroxidase activity of mitochondrial membrane protein cytochrome c (cyt c) by perturbing its tertiary structure in the presence of surface-functionalised gold nanoparticles (GNPs) within cetyltrimethylammonium bromide (CTAB) reverse micelles. The loss in the tertiary structure of cyt c exposes its heme moiety (which is buried inside in the native globular form), which provides greater substrate (pyrogallol and H2O2) accessibility to the reactive heme residue. The surfactant shell of the CTAB reverse micelle in the presence of co-surfactant (n-hexanol) exerted higher crowding effects on the interfacially bound cyt c than similar anionic systems. The congested interface led to protein unfolding, which resulted in a 56-fold higher peroxidase activity of cyt c than that in water. Further perturbation in the protein's structure was achieved by doping amphiphile-capped GNPs with varying hydrophobicities in the water pool of the reverse micelles. The hydrophobic moiety on the surface of the GNPs was directed towards the interfacial region, which induced major steric strain at the interface. Consequently, interaction of the protein with the hydrophobic domain of the amphiphile further disrupted its tertiary structure, which led to better opening up of the heme residue and, thereby, superior activity of the cyt c. The cyt c activity in the reverse micelles proportionately enhanced with an increase in the hydrophobicity of the GNP-capping amphiphiles. A rigid cholesterol moiety as the hydrophobic end group of the GNP strikingly improved the cyt c activity by up to 200-fold relative to that found in aqueous buffer. Fluorescence studies with both a tryptophan residue (Trp59) of the native protein and the sodium salt of fluorescein delineated the crucial role of the hydrophobicity of the GNP-capping amphiphiles in improving the peroxidase activity of cyt c by unfolding its tertiary structure within the reverse micelles.
Effects of Single-Stranded n-Alkyl Amphiphiles on the Conformational and Dynamic Behavior of Lecithin Sonicated Bilayers and Micelles Studied by 13C NMR. A Measure of Lipid Resistance against Disruption of the Bilayer Orientation
Haan, Jan W. de,Weerd, Roel J. E. M. de,Ven, Leo J. M. van de,Buck, Henk M.
, p. 5093 - 5099 (1984)
The triplet fine structures in the 13C NMR spectra of carbons in the ?-position to nitrogen in several n-alkyltrimethylammonium bromides (TAB's), DMPC, and DPPC have been studied in different aggregational states under conditions of enhanced proton noise decoupling.Under the same conditions, the signals of the hydrophobic tails of the lecithins could also be studied better than hitherto, mainly by virtue of relatively narrow signals.It is shown that relatively small changes in packing and lateral motions can be detected.Intercalation of several n-alkyltrimethylammonium bromides in lecithin vesicles causes no decrease of the lipid -N+(CH3)3 mobility around the CH2-CH2 head-group linkage nor a decrease in acyl chain mobility.Moreover, no changes in acyl chain kinking are detected.On the other hand, the incorporated TAB molecules are forced by the lecithin molecules toward severely restricted head-group and tail mobilities.For those combinations of PC and TAB's where the TAB 13C NMR signals are detectable, a change in the conformational equilibrium toward more extension is found.A packing model for the incorporation of TAB's in PC vesicles is presented which probably has a rather general validity.The behavior of mixed micelles of PC's and TAB's, originating from enhancing the latter's concentration, is also described.In these systems, mobilities and chain kinking are increased with respect to the vesicular state.
GEMINI TYPE SURFACTANT
-
Paragraph 0094; 0095, (2017/05/17)
PROBLEM TO BE SOLVED: To provide a gemini type surfactant that has a novel structure and can be synthesized conveniently. SOLUTION: The present invention provides a gemini type surfactant represented by formula (1), where R1-R5 independently represent alkyl, hydroxyalkyl, aryl or the like; R is alkyl, hydroxyalkyl, aryl or the like; Y1 and Y2 independently represent alkylene; n is an integer of 2-4; A- is a counterion; where, R1-R2 and R3-R4 must be the same combination; Z is NR5 or O; R5 is an alkyl group, hydroxyalkyl, aryl or the like. SELECTED DRAWING: None COPYRIGHT: (C)2017,JPOandINPIT
Gemini type surfactant
-
Paragraph 0070, (2017/01/02)
PROBLEM TO BE SOLVED: To provide a gemini type surfactant being a compound having two hydrophilic groups and two hydrophobic groups that has a novel structure and can be simply synthesized. SOLUTION: The gemini type surfactant has a structure represented by formula (1). [In formula (1), R1 to R4 are each independently a 1-20C alkyl group, a 1-20C hydroxyalkyl group, a 1-20C halogenated alkyl group or a 6-20C aromatic hydrocarbon group; Z is N or O atom; Y1 and Y2 are each independently a 1-6C alkylene group; n is an integer of 0 to 2; m is 0 or 1; A- is a counter ion; and provided that any two of R1 to R5 may be bonded to each other to form a ring structure.] COPYRIGHT: (C)2015,JPOandINPIT
Kinetics of hydrolysis of procaine in aqueous and micellar media
Al-Blewi, Fawzia Faleh,Al-Lohedan, Hamad A.,Rafiquee,Issa, Zuheir A.
, p. 1 - 9 (2013/01/15)
The kinetics of alkaline hydrolysis of procaine under the pseudo-first-order condition ([OH-] a [procaine]) has been carried out. N,N-Diethylaminoethanol and p-aminobenzoate anion were obtained as the hydrolysis product. The rate of hydrolysis was found to be linearly dependent upon [NaOH]. The addition of cationic cetyltrimethylammonium bromide (CTAB), dodecyltrimethylammonium bromide (DDTAB) and tetradecyltrimethylammonium bromide, and anionic sodium dodecyl sulfate (SDS) micelles inhibited the rate of hydrolysis. The maximum inhibitive effect on the reaction rate was observed for SDS micelles, whereas among the cationic surfactants, CTAB inhibited most. The variation in the rate of hydrolysis of procaine in the micellar media is attributed to the orientation of a reactive molecule to the surfactant and the binding constant of procaine with micelles. The rate of hydrolysis of procaine is negligible in DDTAB micelles. The observed results in the presence of cationic micelles were treated on the basis of the pseudophase ion exchange model. The results obtained in the presence of anionic micelles were treated by the pseudophase model, and the various kinetic parameters were determined.
1119-94-4 Process route
-

-
4-(4-Dimethylamino-phenylazo)-benzenesulfonatedodecyl-trimethyl-ammonium;

-

- 547-58-0
methyl orange

-

- 1119-94-4
dodecyltrimethylammonium bromide
| Conditions | Yield |
|---|---|
|
With sodium bromide; at 19.9 ℃; Equilibrium constant; var. temps.;
|
-

- 143-15-7
1-dodecylbromide

-

- 75-50-3
trimethylamine

-

- 1119-94-4
dodecyltrimethylammonium bromide
| Conditions | Yield |
|---|---|
|
In acetonitrile; at 80 ℃;
|
80% |
|
In acetonitrile; at 80 ℃;
|
80% |
|
With ethanol;
|
|
|
|
|
|
In ethanol;
|
|
|
In acetonitrile; for 48h;
|
|
|
In isopropyl alcohol; for 48h; Reflux;
|
|
|
In isopropyl alcohol; for 48h; Reflux;
|
1119-94-4 Upstream products
-
143-15-7

1-dodecylbromide
-
75-50-3

trimethylamine
-
74-83-9

methyl bromide
-
112-18-5

N,N-dimethylaminododecane
1119-94-4 Downstream products
-
74-83-9

methyl bromide
-
95-16-9

1,3-Benzothiazole
-
6340-31-4

2-undecylbenzo[d]thiazole
Relevant Products
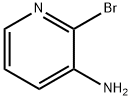
-
3-Amino-2-bromopyridineCAS NO.: 39856-58-1
CAS:39856-58-1
-
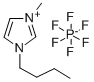
1H-Imidazolium, 3-butyl-1-methyl-, hexafluorophosphate(1-) (1:1)
CAS:174501-64-5
-
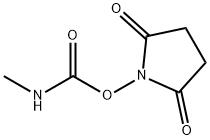
2,5-Pyrrolidinedione,1-[[(methylamino)carbonyl]oxy]-
CAS:18342-66-0