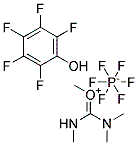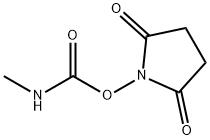
18342-66-0
- Product Name:2,5-Pyrrolidinedione,1-[[(methylamino)carbonyl]oxy]-
- Molecular Formula:C6H8N2O4
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 18342-66-0
Molecular Formula: C6H8N2O4
18342-66-0 Properties
- Molecular Formula:C6H8N2O4
- Molecular Weight:172.141
- Melting Point:148-153 °C
- Refractive Index:1.527
- PKA:11.00±0.46(Predicted)
- PSA:75.71000
- Density:1.416 g/cm3
- LogP:-0.26480
18342-66-0 Usage
Uses
N-Succinimidyl N-Methylcarbamate is used as a reagent in the synthesis of Argifin, a natural product chitinase inhibitor with chemotherapeutic potential. N-Succinimidyl N-Methylcarbamate is also used as a reagent in the preparation of 5-Amino-N1-methyl-1H-imidazole-1,4-dicarboxamide (A616300); a metabolite of Temozolomide (T017775) which is an imidazotetrazine alkylating agent and antineoplastic.
InChI:InChI=1/C6H8N2O4/c1-7-6(11)12-8-4(9)2-3-5(8)10/h2-3H2,1H3,(H,7,11)
18342-66-0 Relevant articles
Synthesis and in vitro antitumor activity of novel 2-alkyl-5- methoxycarbonyl-11-methyl-6H-pyrido[4,3-b]carbazol-2-ium and 2-alkylellipticin-2-ium chloride derivatives
Mori, Ryota,Kato, Asako,Komenoi, Kousuke,Kurasaki, Haruaki,Iijima, Touru,Kawagoshi, Masashi,Kiran,Takeda, Sho,Sakai, Norio,Konakahara, Takeo
, p. 16 - 35 (2014/06/09)
Twenty-one types of novel ellipticine derivatives and pyridocarbazoles (5-methoxycarbonyl-11-methyl-6H-pyrido[4,3-b]carbazoles) with a nitrosourea moiety, linked by an oxydiethylene unit at the 2 position, were synthesized, and their cytotoxicity against HeLa S-3 cells was evaluated. Some of these new compounds exhibited potent antitumor activity by comparison with that of ellipticine.
Preparation and screening against acetylcholinesterase of a non-peptide "indexed" combinatorial library
Pirrung, Michael C.,Chen, Jrlung
, p. 1240 - 1245 (2007/10/02)
A combinatorial library composed from nine alcohols and six isocyanates to formally generate 54 carbamates has been designed prepared, and screened against acetylcholinesterase from the electric eel. In order to deduce the most active member of the library, it was prepared as 15 sublibraries in which one of the reacting components was fixed and the other reactants were used as an equimolar mixture. The product mixtures were tested and their activities used as "indices" to the rows or columns of a two-dimensional matrix reflecting the activities of individual carbamates. A number of carbamates in the most active row and column were synthesized and assayed, demonstrating that the most active cell in the matrix could be identified by the sublibrary synthesis procedure. Other methods for generating large libraries of molecules for biological screening that have recently been developed have relied on a covalent attachment between library members and a label to identify the active components. Indexed libraries otter the advantage that they can be prepared from any class of compounds composed from multiple subunits and that any type of assay (binding, enzyme inhibition, agonism/antagonism, cell-based, or even whole organism assays for biological activity) can be used because all compounds are generated in a free form.
A convenient method for the synthesis of activated N-methylcarbamates
Konakahara,Ozaki,Sato,Gold
, p. 103 - 106 (2007/10/02)
An investigation of methods to efficiently prepare activated N-methylcarbamates is reported. N-(Methylcarbamoyloxy)succinimide (3a), aryl N-methylcarbamates 3b-d and 2,2,2-trifluoro-1-(trifluoromethyl)ethyl N-methylcarbamate (3e) have been prepared in 70-80% yields from the corresponding chloroformates 5a-e, which were prepared as crystalline solids by the condensation of trichloromethylchloroformate (1) or bis(trichloromethyl)carbonate (2) with hydroxy compounds 4a-e in high yields.
Convenient Methods for Syntheses of Active Carbamates, Ureas and Nitrosoureas Using N,N'-disuccinimido Carbonate (DSC)
Takeda, Kazuyoshi,Akagi, Yoshie,Saiki, Atsuko,Tsukahara, Toshiko,Ogura, Haruo
, p. 4569 - 4572 (2007/10/02)
The reaction of DSC and amino compounds afforded the corresponding carbamates which were able to be converted into ureas and nitrosoureas.
18342-66-0 Process route
-

- 6066-82-6
1-hydroxy-pyrrolidine-2,5-dione

-

- 624-83-9
methyl isocyanate

-

- 18342-66-0
2,5-dioxopyrrolidin-1-yl methylcarbamate
| Conditions | Yield |
|---|---|
|
With triethylamine; In ethyl acetate; at 0 - 20 ℃; for 24h;
|
86% |
|
With pyridine; for 24h; Ambient temperature;
|
80% |
|
With triethylamine; In tetrahydrofuran; 1.) 60-80 deg C, 4 h, 2.) RT, overnight;
|
-

- 15149-73-2
2,5-dioxopyrrolidin-1-yl carbonochloridate

-

- 593-51-1
methylamine hydrochloride

-

- 18342-66-0
2,5-dioxopyrrolidin-1-yl methylcarbamate
| Conditions | Yield |
|---|---|
|
With N-ethyl-N,N-diisopropylamine; In dichloromethane; at 0 - 3 ℃; for 48h;
|
80% |
18342-66-0 Upstream products
-
6066-82-6

1-hydroxy-pyrrolidine-2,5-dione
-
624-83-9

methyl isocyanate
-
15149-73-2

2,5-dioxopyrrolidin-1-yl carbonochloridate
-
593-51-1

methylamine hydrochloride
18342-66-0 Downstream products
-
80354-48-9

N-methyl-N-nitrosocarbamic acid N'-hydroxysuccinimide ester
-
243975-37-3

cyclo(Nw-(N-methylcarbamoyl)-L-arginyl-N-methyl-L-phenylalanyl-β-L-aspartyl-β-L-aspartyl-D-alanyl)
-
57-47-6

(+/-)-Physostigmine
-
857275-30-0

benzyl (3R,4R)-3-{7-bromo-3-methyl-1-[2-(3-methylureido)ethyl]-1H-indol-6-ylmethoxy}-4-{4-[3-(2-methoxybenzyl)oxypropoxy]phenyl}piperidine-1-carboxylate
Relevant Products
-
1-Dodecanaminium,N,N,N-trimethyl-, bromide (1:1)
CAS:1119-94-4
-
Pyridinium,1-hexadecyl-, chloride, hydrate (1:1:1)
CAS:6004-24-6