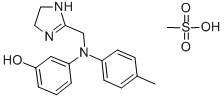
65-28-1
- Product Name:Phentolamine mesilate
- Molecular Formula:C18H23N3O4S
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 65-28-1
Molecular Formula: C18H23N3O4S
Appearance: white to off-white crystalline solid
65-28-1 Properties
- Molecular Formula:C18H23N3O4S
- Molecular Weight:377.464
- Appearance/Colour:white to off-white crystalline solid
- Vapor Pressure:9.48E-13mmHg at 25°C
- Melting Point:180-182 °C
- Refractive Index:1.6320 (estimate)
- Boiling Point:551 °C at 760 mmHg
- Flash Point:287 °C
- PSA:110.61000
- Density:1.2256 (rough estimate)
- LogP:3.18960
65-28-1 Usage
Chemical Properties
Crystalline Solid
Uses
Phentolamine Mesylate is a nonselective alpha-adrenergic antagonist with IC50 of 0.1 μM
Uses
Phentolamine Methanesulfonate Salt is an adrenergic blocking agent. An antihypertensive. Used for the treatment of pheochromocytoma.
Uses
As an adrenergic blocking agent and an antihypertensive, Phentolamine mesilate can be used for the treatment of pheochromocytoma.
Brand name
Regitine (Novartis) [Name previously used: Phentolamine Methanesulfonate.].
General Description
Phentolamine Mesylate belongs to the class of alpha adrenergic antagonist drugs. It is used in the treatment of pheochromocytoma-related hypertension, erectile dysfunction and in the diagnosis of pheochromocytoma and norepinephrine-related dermal necrosis.Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Biochem/physiol Actions
α-Adrenergic receptor antagonist; peripheral vasodilator.
Clinical Use
Alpha-adrenoceptor blocker: Hypertensive crisis
Safety Profile
Poison by intravenous and subcutaneous routes. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of SOx and NOx.
Drug interactions
Potentially hazardous interactions with other drugs Anaesthetics: enhanced hypotensive effect. Antidepressants: additive hypotensive effect with MAOIs - avoid. Antihypertensives: enhanced hypotensive effect. Avanafil, vardenafil, sildenafil and tadalafil: enhanced hypotensive effect - avoid concomitant use. Diuretics: enhanced hypotensive effect. Linezolid: additive hypotensive effect. Moxisylyte: possibly severe postural hypotension.
Metabolism
Phentolamine is extensively metabolised. Only about 10-13% of an intravenous dose is excreted unchanged in the urine, and the fate of the remainder of the drug is unknown.
InChI:InChI=1/C17H19N3O.CH4O3S/c1-13-5-7-14(8-6-13)20(12-17-18-9-10-19-17)15-3-2-4-16(21)11-15;1-5(2,3)4/h2-8,11,21H,9-10,12H2,1H3,(H,18,19);1H3,(H,2,3,4)
65-28-1 Relevant articles
Process for synthesis of phentolamine mesylate (by machine translation)
-
Paragraph 033; 0034; 0035; 0036, (2016/10/07)
The invention discloses a synthesis process of phentolamine mesilate. The synthesis process of the phentolamine mesilate comprises the following steps: A, neutralizing, namely, adjusting phentolamine hydrochloride by using diluted ammonia water for many times under the action of silica gel until proper pH value is achieved, and filtering to obtain filtrate to obtain phentolamine free alkali, watering and washing with distilled water for many times until the filtrate is qualified through chloride tracking detection, and drying and crystallizing to obtain a phentolamine free alkali refined product; and B, salifying namely, adding an ethanol solution of methanesulfonic acid into the phentolamine free alkali refined product dropwise by taking absolute ethanol as a solvent, precisely adjusting the pH value to be 5 to 6, then performing a water bath vacuum distillation salt forming reaction, inoculating ethyl acetate, separating out and crystallizing, washing and drying to obtain the phentolamine mesilate. According to the synthesis process of the phentolamine mesilate, the prominent problems that chloride and pH value are not easy to control during actual production are solved, purity is high and impurities are few.
65-28-1 Process route
-

- 75-75-2,98527-29-8
methanesulfonic acid

-

- 50-60-2
phentolamine

-

- 65-28-1
phentolamine mesylate
| Conditions | Yield |
|---|---|
|
In ethanol; pH=5 - 6;
|
99% |
-

-
N-(quinolin-8-yl)but‐3‐enamide

-

- 65-28-1
phentolamine mesylate

-

-
2-(((3-hydroxy-4-(4-oxo-4-(quinolin-8-ylamino)butyl)phenyl)(p-tolyl)amino)methyl)-4,5-dihydro-1Himidazol-3-ium acetate
| Conditions | Yield |
|---|---|
|
With palladium diacetate; acetic acid; In acetonitrile; at 120 ℃; for 4h;
|
33% |
65-28-1 Upstream products
-
75-75-2

methanesulfonic acid
-
50-60-2

phentolamine
Relevant Products
-
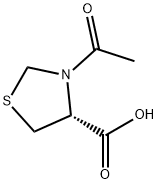
N-Acetyl-L-thioprolineCAS NO.: 54323-50-1
CAS:54323-50-1
-
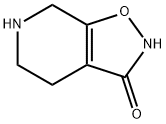
Isoxazolo[5,4-c]pyridin-3(2H)-one,4,5,6,7-tetrahydro-
CAS:64603-91-4
-
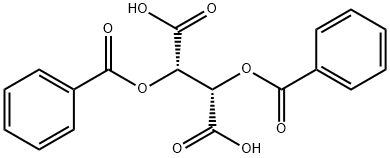
(+)-Dibenzoyl-D-tartaric acid
CAS:17026-42-5