17026-42-5
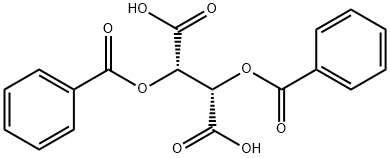
- Product Name:(+)-Dibenzoyl-D-tartaric acid
- Molecular Formula:C18H14O8
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 17026-42-5
Molecular Formula: C18H14O8
Appearance: white to light yellow crystal powder
17026-42-5 Properties
- Molecular Formula:C18H14O8
- Molecular Weight:358.304
- Appearance/Colour:white to light yellow crystal powder
- Melting Point:154-156 ºC (lit.)
- Refractive Index:1.667
- Boiling Point:606.6 ºC at 760 mmHg
- PKA:1.85±0.25(Predicted)
- Flash Point:221.8 ºC
- PSA:127.20000
- Density:1.438 g/cm3
- LogP:1.60680
17026-42-5 Usage
Chemical Properties
white to light yellow crystal powde
Uses
(+)-Dibenzoyl-D-tartaric acid is a reagent used to produce chiral salts.
Uses
For chiral resolution(+)-Dibenzoyl-D-tartaric acid is used as a reagent in the preparation of chiral salts. It also serves as a reagent for chiral resolution of amino compounds.
General Description
(+)-2,3-Dibenzoyl-D-tartaric acid is commonly used as an optically active resolving agent in the chiral resolution process such as diastereomeric salt resolution technique.
InChI:InChI=1/C18H14O8/c19-13(11-7-3-1-4-8-11)17(25,15(21)22)18(26,16(23)24)14(20)12-9-5-2-6-10-12/h1-10,25-26H,(H,21,22)(H,23,24)/t17-,18-/m0/s1
17026-42-5 Relevant articles
New access to enantiopure O,O'-dibenzoyltartaric acid: Resolution of the mixed calcium methoxyacetate by preferential crystallization
Elekes, Ferenc,Kovari, Zoltan,Mravik, Andras,Boecskei, Zsolt,Fogassy, Elemer
, p. 2895 - 2900 (1998)
A convenient resolution of racemic O,O'-dibenzoyltartaric acid by preferential crystallization of its mixed calcium salt formed with methoxyacetic acid is described. The separation of the partially prepared salt 2 followed by a subsequent recrystallization results in a simple and effective resolution of the title compound during which pure enantiomers of 1 can be obtained even on a large scale. The crystal structure of the conglomerate forming complex 2 is also reported.
Preparation method of (1R,2S)-1-phenyl-2-(1-pyrrolidyl)-1-propanol
-
Paragraph 0033, (2016/10/31)
The invention discloses a preparation method of (1R,2S)-1-phenyl-2-(1-pyrrolidyl)-1-propanol. According to the preparation method, DL-1-phenyl-2-(1-pyrrolidyl)-1-acetone is taken as a starting material and subjected to resolution, racemization and reduction, and (1R,2S)-1-phenyl-2-(1-pyrrolidyl)-1-propanol is prepared. The yield of one-time resolution is higher than 35%, a resolving agent is easy to recover, and the recovery rate is higher than 90%; the racemization process is performed under the slightly alkaline condition, and the racemization yield is higher; the yield of (1R,2S)-1-phenyl-2-(1-pyrrolidyl)-1-propanol obtained through reduction is higher than 85%. The preparation method has the advantages of mild reaction conditions, stable process, high product optical purity, low cost, high production safety and the like.
New chiral zwitterionic phosphorus heterocycles: Synthesis, structure, properties and application as chiral solvating agents
Sheshenev, Andrey E.,Boltukhina, Ekaterina V.,Grishina, Anastasiya A.,Cisa?ova, Ivana,Lyapkalo, Ilya M.,Hii, King Kuok
, p. 8136 - 8143 (2013/07/27)
A family of new chiral zwitterionic phosphorus-containing heterocycles (zPHC) have been derived from methylene-bridged bis(imidazolines). These structures were unambiguously determined, including single-crystal XRD analysis for two compounds. The stability, acid/base and electronic properties of these dipolar phosphorus heterocycles were subsequently investigated. zPHCs can be successfully employed as a new class of chiral solvating agents for the enantiodifferentiation of chiral carboxylic and sulfonic acids by NMR spectroscopy. The stoichiometry and binding constants for the donor-acceptor complexes formed were established by NMR titration methods. A convenient synthetic approach to a new class of chiral zwitterionic phosphorus-containing heterocycles starting from methylene-bridged bis(imidazolines) was designed and executed. Stability and properties of the synthesized compounds were investigated. The applicability of the designed compounds as chiral solvating agents for the determination of the enantiomeric excesses of chiral acids was demonstrated. Copyright
PROCESS FOR THE RESOLUTION OF (R,S)-NICOTINE
-
Page/Page column 3, (2012/08/08)
(R,S)-Nicotine was resolved through diastereomeric salt formation using dibenzoyl-d-tartaric acid and dibenzoyl-l-tartaric acid to obtain enantiomerically pure (S)-nicotine and (R)-nicotine.
COMPOSITIONS AND METHODS FOR CYCLOFRUCTANS AS SEPARATION AGENTS
-
Page/Page column 45-49; 51, (2010/12/31)
The present invention relates to derivatized cyclofructan compounds, compositions comprising derivatized cyclofructan compounds, and methods of using compositions comprising derivatized cyclofructan compounds for chromatographic separations of chemical species, including enantiomers. Said compositions may comprise a solid support and/or polymers comprising derivatized cyclofructan compounds.
17026-42-5 Process route
-

-
S-nicotine dibenzoyl-d-tartrate

-

- 17026-42-5
O,O'-dibenzoyl-D-tartaric acid

-

- 54-11-5
nicotin
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; for 0.166667h; Product distribution / selectivity;
|
65.5% |
-

-
L-1-phenyl-2-(1-pyrrolidinyl)-1-propanone-(-)-dibenzoyl-L-tartaric acid salt

-

- 19134-50-0
(SR)-(+/-)-1-phenyl-2-(1-pyrrolidinyl)-1-propanone

-

- 17026-42-5
O,O'-dibenzoyl-D-tartaric acid
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; sodium hydroxide; In water; ethyl acetate; at 80 ℃; for 5h; pH=2 - 11.5;
|
87.6% |
17026-42-5 Upstream products
-
87-69-4

L-Tartaric acid
-
98-88-4

benzoyl chloride
-
147-71-7

D-tartaric acid
-
2743-38-6

(+/-)-2,3-O-dibenzoyltartaric acid
17026-42-5 Downstream products
-
140427-67-4

dimethyl 2-hydroxybutyl sulfonium dibenzoyltartrate
-
140427-53-8

2-hydroxybutyl methyl phenyl sulfonium dibenzoyltartrate
-
131101-16-1

(2S,3S)-2,3-Bis-benzoyloxy-succinic acid; compound with (S)-3-(2-amino-ethyl)-5-methoxy-1,3-dimethyl-1,3-dihydro-indol-2-one
-
140630-10-0

dimethyl (2-hydroxy-3-octadecyloxypropyl) sulfonium dibenzoyltartrate
Relevant Products
-
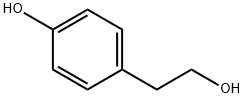
4-Hydroxyphenethyl alcoholCAS NO.: 501-94-0
CAS:501-94-0
-
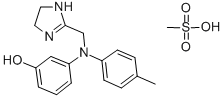
Phentolamine mesilate
CAS:65-28-1
-
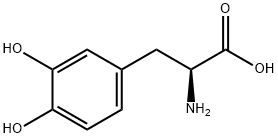
Levodopa
CAS:59-92-7