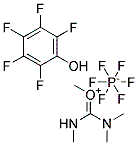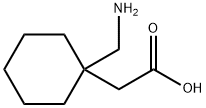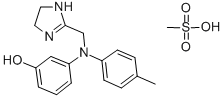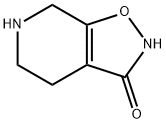
64603-91-4
- Product Name:Isoxazolo[5,4-c]pyridin-3(2H)-one,4,5,6,7-tetrahydro-
- Molecular Formula:C6H8 N2 O2
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 64603-91-4
Molecular Formula: C6H8 N2 O2
Appearance: white solid
64603-91-4 Properties
- Molecular Formula:C6H8 N2 O2
- Molecular Weight:140.142
- Appearance/Colour:white solid
- Vapor Pressure:0.00113mmHg at 25°C
- Melting Point:242-244° (dec)
- Boiling Point:340.5 °C at 760 mmHg
- PKA:pKa (water, 25°): 4.44 ±0.03; 8.48 ±0.04
- Flash Point:159.7 °C
- PSA:58.29000
- Density:1.36 g/cm3
- LogP:1.15670
64603-91-4 Usage
Description
Gaboxadol HCl (64603-91-4) is a GABA-A receptor agonist. Hypnotic agent.
Uses
GABAa agonist, GABAc antagonist
Biological Activity
Systemically active GABA A receptor agonist and GABA C receptor antagonist. Displays antinociceptive, anticonvulsant and sedative effects. Hypnotic agent that enhances delta activity within non-REM sleep in rats.
References
1) Meera et al. (2011), Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA(A) receptors; J. Neurophysiol., 106 2057
InChI:InChI=1/C6H8N2O2.ClH/c9-6-4-1-2-7-3-5(4)10-8-6;/h7H,1-3H2,(H,8,9);1H
64603-91-4 Relevant articles
GABOXADOL FORMS, COMPOSITIONS THEREOF, AND RELATED METHODS
-
Page/Page column 27, (2008/06/13)
The invention provides novel gaboxadol forms. These forms include salts, hydrates, solvates, and polymorphs of gaboxadol. The invention also provides novel compositions comprising these novel soluble forms and a suitable carrier. The invention also provides related methods of treatment. Compositions and methods of the invention of the invention have a number of uses, including the treatment or prevention of sleep disorders.
Polymorphic forms of a GABAA agonist
-
Page/Page column 5, (2008/06/13)
Two new crystalline monhydrates and two new crystalline anhydrates of gaboxadol are disclosed together with methods for preparing them.
Heterocyclic compounds
-
, (2008/06/13)
The compound Ia STR1 has been shown to possess GABA-related activity. The invention relates to Ia and derivatives thereof, covered by the formula STR2 in which R" is hydrogen, acetyl or a group of the general formula STR3 in which R5 is C1-8 alkyl; phenyl; phenyl substituted in the 4-position with halogen, lower alkoxy, or lower alkyl; or phenylalkyl in which the phenyl group may be substituted in the 4-position with halogen, lower alkoxy, or lower alkyl; and salts thereof. Novel intermediates for preparing I are STR4 in which Alk is a lower alkyl group and Z is hydrogen or an amino-protecting group; STR5 wherein Z is hydrogen or an amino-protecting group, T is a group convertible, by hydrolysis, into an oxy group, and Q is a leaving group which, on reaction with hydroxylamine, forms a hydroxamic acid group; STR6 wherein Z and T are as defined above; STR7 wherein Z is as defined above, and W is hydrogen or a group removable to yield the free hydroxy group, with the proviso that at least one of Z and W is different from hydrogen.
64603-91-4 Process route
-
![3-hydroxy-4,5,6,7-tetrahydro-1,2-oxazolo-[5,4-c]pyridin-6-ium chloride](/upload/2023/1/a63b8cea-812a-4667-bba7-9c77e55b3c49.png)
-
3-hydroxy-4,5,6,7-tetrahydro-1,2-oxazolo-[5,4-c]pyridin-6-ium chloride

-

- 64603-91-4
4,5,6,7-tetrahydroisoxazole<5,4-c>pyridin-3-ol
| Conditions | Yield |
|---|---|
|
With triethylamine; In ethanol; water; at 5 ℃; for 2h;
|
-
![4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol monohydrate](/upload/2023/1/5a070f09-9569-4678-8b38-c6d6a986bda4.png)
- 815574-58-4
4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol monohydrate

-

- 64603-91-4
4,5,6,7-tetrahydroisoxazole<5,4-c>pyridin-3-ol
| Conditions | Yield |
|---|---|
|
at 110 ℃; for 1.25h; under 760.051 Torr;
|
|
|
at 110 ℃; for 1.25h; under 760.051 Torr;
|
64603-91-4 Upstream products
-
815574-58-4

4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol monohydrate
-
65202-62-2

methyl 3-hydroxy-4,5,6,7-tetraydroisoxazolo<5,4-c>pyridine-6-carboxylate
64603-91-4 Downstream products
-
914291-73-9

gaboxadol, N,N-dibenzylethylenediamine salt
Relevant Products
-
Gabapentin hydrochloride
CAS:60142-96-3
-
Phentolamine mesilate
CAS:65-28-1