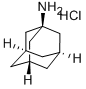
665-66-7
- Product Name:1-Adamantanamine hydrochloride
- Molecular Formula:C10H18ClN
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 665-66-7
Molecular Formula: C10H18ClN
Appearance: Crystalline solid
665-66-7 Properties
- Molecular Formula:C10H18ClN
- Molecular Weight:187.713
- Appearance/Colour:Crystalline solid
- Vapor Pressure:0.085mmHg at 25°C
- Melting Point:>300 °C(lit.)
- Refractive Index:1.558
- Boiling Point:1-Adamantanamine hydrochloride
- Flash Point:96 °C
- PSA:26.02000
- Density:1.067g/cm3
- LogP:3.41620
665-66-7 Usage
Chemical Properties
Crystalline Solid
Originator
Symmetrel,DuPont (Endo),US,1966
Uses
1-Adamantanamine hydrochloride is used in the prophylactic or symptomatic treatment of influenza A. It is also used as an antiparkinsonian agent, to treat extra pyramidal reactions, and for postherpetic neuralgia. It is also used an NMDA-receptor antagoinst.
Uses
antiviral, antiparkinsonian; treatment of drug-induced extrapyrimidal reactions
Uses
selective FP prostanoid receptor agonist, F-series prostaglandin analog. 200 times as potent as Latanoprost -20oC
Uses
NMDA-receptor antagonist. Antiviral; antiparkinsonian
Definition
ChEBI: A hydrochloride obtained by combining amantadine and hydrochloric acid in equimolar amounts.
Manufacturing Process
360 ml of 96% sulfuric acid and a solution of 13.6 grams (0.1 mol) of adamantane in 100 ml of n-hexane were emulsified in the apparatus described and provided with an inclined centrifugal stirrer. Then a mixture of 46 grams (1.7 mols) of liquid hydrocyanic acid and 29.6 grams (0.4 mol) of tertiary butanol was added dropwise within 1.5 hours at about 25°C. After 30 minutes of postreaction, the product was poured on ice. The granular mass which precipitated [N-(adamantyl-1)formamide] was sucked off and washed with water. The raw product (37 grams) was then refluxed for 10 hours with a solution of 60 grams of NaOH in 600 ml of diethylene glycol. After cooling, the solution was diluted with 1.5 liters of water and subjected to three extractions with ether. The amine was extracted from the ethereal solution with 2 N HCl and liberated therefrom by the addition of solid NaOH (while cooling). The alkaline solution was extracted with ether and the ethereal solution was dried with solid NaOH. Distillation resulted in 10.6 grams (70% of the theory) of 1-aminoadamantane which, after sublimation, melted at 180°C to 192°C (seal capillary). It is converted to the hydrochloride.
Brand name
Symadine (Solvay Pharmaceuticals); Symmetrel (Endo).
Therapeutic Function
Antiviral, Antiparkinsonian
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Biological Activity
amantadine hydrochloride is an antiviral and an antiparkinsonian drug.
Biochem/physiol Actions
Amantadine hydrochloride is effective against influenza viruses both in vivo and in vitro. It is considered as an antagonist of the N-methyl-D-aspartate (NMDA) type glutamate receptor. Amantadine plays an important role in the release of dopamine, preventing dopamine reuptake and blocking microglial activation and neuroinflammation.
Clinical Use
Parkinson’s disease (but not drug-induced extrapyramidal symptoms) Post-herpetic neuralgia Prophylaxis and treatment of influenza A
Safety Profile
Human poison by ingestion. Poison by ingestion, intraperitoneal, and intravenous routes. A human teratogen with developmental abnormalities of the circulatory system. Experimental reproductive effects. Human systemic effects by ingestion: distorted perceptions, euphoria, excitement, hallucinations. When heated to decomposition it emits very toxic fumes of NO, and HCl.
Drug interactions
Potentially hazardous interactions with other drugs Memantine: increased risk of CNS toxicity - avoid; effects of amantadine possibly enhanced.
Metabolism
Amantadine is metabolised in the liver to a minor extent, mainly by N-acetylation. The renal amantadine clearance is much higher than the creatinine clearance, suggesting renal tubular secretion in addition to glomerular filtration. After 4-5 days, 90% of the dose appears unchanged in urine. The rate is considerably influenced by urinary pH: a rise in pH brings about a fall in excretion.
Purification Methods
Dissolve the salt in dry EtOH, add a few drops of dry EtOH saturated with HCl gas, followed by dry Et2O to crystallise the hydrochloride. Dry the salt in a vacuum. [Stetter et al. Chem Ber 93 226 1960.]
InChI:InChI=1/C10H17N/c11-10-4-7-1-8(5-10)3-9(2-7)6-10/h7-9H,1-6,11H2/t7-,8+,9-,10-
665-66-7 Relevant articles
Preparation method of amantadine
-
Paragraph 0093-0107, (2021/06/13)
The invention discloses a preparation method of amantadine, which belongs to the technical field of organic chemical synthesis, and is characterized by comprising the following steps: taking a compound adamantane as an initial raw material, generating an intermediate 1-acetamido adamantane in the presence of acetonitrile, a polyion liquid PIL catalyst and sulfuric acid, and then hydrolyzing the intermediate into amantadine in a system of alcohol and alkali. The preparation method is environment-friendly, post-treatment is convenient, the use amount of sulfuric acid and acetonitrile is greatly reduced through the catalyst polyion liquid, and post-treatment is simple. The ionic liquid catalyst can be recycled, so that the cost is greatly saved, and the method is suitable for large-scale industrial production.
Amantadine hydrochloride and preparation method thereof
-
, (2021/03/18)
The invention discloses amantadine hydrochloride and a preparation method thereof, and relates to the technical field of amantadine hydrochloride synthesis. The method aims at solving the problems that the reaction time is too long and the yield of amantadine hydrochloride is not high. The amantadine hydrochloride is prepared from the following components, by weight: 5mmol of nitro compound, 5g ofcatalyst, 15ml of absolute ethyl alcohol, 15ml of concentrated hydrochloric acid, 15ml of hydrazine hydrate and 3ml of sodium hydroxide solution, The preparation method of amantadine hydrochloride comprises the following steps: respectively adding a nitro compound, absolute ethyl alcohol and a catalyst into a 50mL flask; in the production process of the hydrazine hydrate catalytic reduction method, no pollution is caused, the yield is high, the reaction conditions are mild, the yield of the nitro compound is 90%, the conversion rate of the nitro compound subjected to hydrazine hydrate catalytic reduction is 98.5%, the yield of the obtained amantadine hydrochloride is 89.5%, and compared with other processes, the yield is higher, the operation is simple, and the efficiency is high.
Cyclic (Alkyl)(amino)carbene Ligand-Promoted Nitro Deoxygenative Hydroboration with Chromium Catalysis: Scope, Mechanism, and Applications
Zhao, Lixing,Hu, Chenyang,Cong, Xuefeng,Deng, Gongda,Liu, Liu Leo,Luo, Meiming,Zeng, Xiaoming
supporting information, p. 1618 - 1629 (2021/01/25)
Transition metal catalysis that utilizes N-heterocyclic carbenes as noninnocent ligands in promoting transformations has not been well studied. We report here a cyclic (alkyl)(amino)carbene (CAAC) ligand-promoted nitro deoxygenative hydroboration with cost-effective chromium catalysis. Using 1 mol % of CAAC-Cr precatalyst, the addition of HBpin to nitro scaffolds leads to deoxygenation, allowing for the retention of various reducible functionalities and the compatibility of sensitive groups toward hydroboration, thereby providing a mild, chemoselective, and facile strategy to form anilines, as well as heteroaryl and aliphatic amine derivatives, with broad scope and particularly high turnover numbers (up to 1.8 × 106). Mechanistic studies, based on theoretical calculations, indicate that the CAAC ligand plays an important role in promoting polarity reversal of hydride of HBpin; it serves as an H-shuttle to facilitate deoxygenative hydroboration. The preparation of several commercially available pharmaceuticals by means of this strategy highlights its potential application in medicinal chemistry.
Synthetic method of amantadine hydrochloride (by machine translation)
-
Paragraph 0115; 0119-0122; 0145; 0149-0156; 0157; 0162-0184, (2020/05/29)
Compared, the prior art . the synthesis method: the amantadine hydrochloride has an important clinical value and market value Ritter, and the synthesis process, of the hydrochloric acid adamantanamine, effectively improves the industrial application potential, of the hydrochloric acid adamantane hydrochloride synthesis process as, by adopting a special process and a component, on the one hand for preparation of the medicine, for treating and preventing viral infection, through hydrolysis and purification on the other hand, and effectively solves the problem, of environmental pollution in the synthesis process of the hydrochloric acid adamantanamine by adopting a special process and a component technology; through hydrolysis and purification on an aspect, through hydrolysis and, purification of components . The method mainly, comprises, steps of preparation of an amantadine hydrochloride synthesis process by using a special process and a method. (by machine translation)
665-66-7 Process route
-

- 281-23-2
adamantane

-

- 665-66-7,13878-11-0
amantadine hydrochloride

-

- 10523-68-9
adamantan-2-amine hydrochloride
| Conditions | Yield |
|---|---|
|
adamantane; With methanol; cerium(III) chloride; di-tert-butyl-diazodicarboxylate; tetrabutyl-ammonium chloride; In acetonitrile; at 20 ℃; for 10h; Irradiation; Inert atmosphere; Sealed tube;
With trifluoroacetic acid; In dichloromethane; at 20 ℃; for 1h; Reagent/catalyst; Further stages;
|
-

- 880-52-4
N-(1-adamantyl)acetamide

-

- 665-66-7,13878-11-0
amantadine hydrochloride
| Conditions | Yield |
|---|---|
|
N-(1-adamantyl)acetamide; With sodium heptadecanoic acid; fipronilβ-cyclodextrin; sodium hydroxide; In ethanol; water; for 20h; Reflux;
With hydrogenchloride; In water; at 70 ℃;
|
96.5% |
|
N-(1-adamantyl)acetamide; With propylene glycol; potassium hydroxide; In water; at 125 - 130 ℃; for 8h; Green chemistry;
With hydrogenchloride; In dichloromethane; water; at 55 - 60 ℃; for 1h; Temperature; Time; Green chemistry;
|
82% |
|
N-(1-adamantyl)acetamide; With methanol; water; sodium hydroxide; at 145 ℃; for 8h; Autoclave;
With hydrogenchloride; In dichloromethane; water; at 50 ℃; for 0.5h; Autoclave;
|
82.2% |
|
N-(1-adamantyl)acetamide; With pyridine; oxalyl dichloride; In tetrahydrofuran; at 0 ℃; for 0.5h; Inert atmosphere;
With propylene glycol; In tetrahydrofuran; at 0 - 25 ℃; Inert atmosphere;
With hydrogenchloride; In ethanol;
|
|
|
N-(1-adamantyl)acetamide; With tetrabutylammomium bromide; sodium hydroxide; In water; ethylene glycol; at 190 ℃; for 12h; Autoclave;
With hydrogenchloride; In water; ethylene glycol; pH=4;
|
|
|
N-(1-adamantyl)acetamide; With tetrabutylammomium bromide; sodium hydroxide; In water; at 190 ℃; for 12h; Autoclave;
With hydrogenchloride; In water; pH=4;
|
|
|
Multi-step reaction with 2 steps
1: sodium hydroxide / 1,2-dimethoxyethane / 12 h / 135 °C
2: hydrogenchloride / dichloromethane; water / 3 h / 50 °C
With hydrogenchloride; sodium hydroxide; In 1,2-dimethoxyethane; dichloromethane; water;
|
665-66-7 Upstream products
-
24375-06-2

1-(Chloroamino)adamantane
-
24886-73-5

1-Azidoadamantane
-
57680-77-0

adamantylmagnesium bromide
-
768-94-5

1-Adamantanamine
665-66-7 Downstream products
-
3716-66-3

1-(2-hydroxyethylamino)adamantane
-
14973-23-0

N-Adamantan-1-yl-N'-<2-fluor-aethyl>-harnstoff
-
57527-54-5

N-(-adamantan-1-yl)-1-phenylmethanimine
-
5689-59-8

N-(1-adamantyl)-2-chloroacetamide
Relevant Products
-
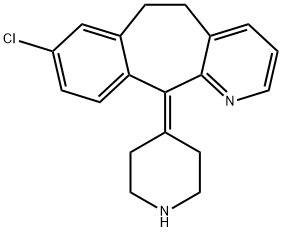
Desloratadine CAS NO.: 100643-71-8
CAS:100643-71-8
-
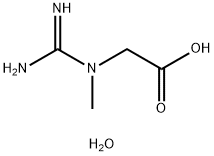
Creatine monohydrate
CAS:6020-87-7
-
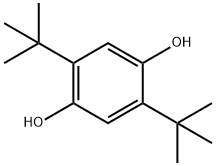
1,4-Benzenediol,2,5-bis(1,1-dimethylethyl)-
CAS:88-58-4