6020-87-7
- Product Name:Creatine monohydrate
- Molecular Formula:C4H9N3O2.H2O
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 6020-87-7
Molecular Formula: C4H9N3O2.H2O
Appearance: white cryst. powder
6020-87-7 Properties
- Molecular Formula:C4H9N3O2.H2O
- Molecular Weight:149.15
- Appearance/Colour:white cryst. powder
- Melting Point:292 °C (dec.)(lit.)
- Boiling Point:271.6 °C at 760 mmHg
- Flash Point:118.1 °C
- PSA:99.64000
- LogP:-0.36800
6020-87-7 Usage
Description
Creatine Monohydrate is the monohydrate form of creatine similar or identical to endogenous creatine produced in the liver, kidneys, and pancreas. Pure creatine is a white, tasteless,odorless powder, that is a naturally occurring metabolite found in muscle tissue.Creatine monohydrate is an amino acid produced in the human body that plays a role in replenishing the energy supply to muscle cells.Creatine is usually produced to a purity of 99.5 percent or higher.Until recently, the primary use for creatine was as a laboratory reagent, demand for which was relatively limited.In the early 1990's, however, weight trainersand other athletes began using creatine in the belief that it stimulates muscle growth and reduces muscle fatigue.
Chemical Properties
Creatine monohydrate is a colorless, crystalline substance used in muscle tissue for the production of phosphocreatine, an important factor in the formation of adenosine triphosphate (ATP), the source of energy for muscle contraction and many other functions in the body.
Uses
Creatine is a natural compound made from the amino acids l-arginine, glycine, and methionine.Creatine monohydrate is a creatine with one molecule of water connected to it. Our bodies can produce creatine, however they also can take in and store creatine found in diverse meals like meat, eggs, and fish.Creatine monohydrate supplementation is promoted as an ergogenic aid, which refers to a product purported to enhance energy production,utilization, control, and efficiency (Mujika and Padilla,1997).Creatine is purported to increase power, strength, and muscle mass and to decrease performance time (Demant et al.,1999).Involved with rapid ATP production primarily in skeletal muscle tissue via the action of creatine kinase(s).
Uses
Creatine monohydrate is involved in rapid ATP production primarily in skeletal muscle tissue via the action of creatine kinase(s). It may be used as a supplement to study its uptake mechanism and metabolism of action. It is used in the treatment of neuromuscular diseases.
Preparation
Creatine monohydrate can be synthesized in the laboratory (and commercially), starting from cyanamide and sarcosine (N-methylglycine).Creatine is a nitrogenous organic acid that occurs naturally in vertebrates and helps to supply energy to all cells in the body, primarily muscle by playing a key role in muscle energy metabolism. It is produced in the liver, pancreas, and kidneys and can also be derived from food and dietary supplements. Creatine provides the energy needed for muscle contraction and substantially improves performance in high-intensity exercise because it improves anaerobic capacity and protein synthesis. Therapeutically, it has been used to treat some types of muscular dystrophy, ocular atrophy, and some types of sclerosis. Creatine (a dietary supplement) is demanded by many athletes and is neither regulated by the Food and Drug Administration (FDA) nor prohibited by the International Olympic Committee (IOC).
benefits
Creatine has many benefits for health and performance. The most common is creatine monohydrate, a dietary supplement that improve strength, increase lean muscle mass, and help the muscles recover more quickly during exercise. It can also inhibit the generation of muscle fatigue factors, reduce fatigue and tension, restore physical fitness, accelerate protein synthesis in the human body, make muscles stronger, enhance muscle elasticity, reduce cholesterol, blood lipids, and blood sugar levels, improve middle-aged and elderly Muscular dystrophy, and delay aging.
Biochem/physiol Actions
Creatine is a nitrogenous compound that acts as a high-energy reservoir for the rapid regeneration of ATP. Approximately 95% of creatine is found in skeletal muscle, primarily as phosphocreatine. Creatine can be acquired through dietary consumption or formed from L-arginine, glycine, and L-methionine in a multi-step reaction that occurs in the kidneys and liver. Creatine is then transported to muscle tissue. Creatine supplementation is used for the enhancement of sports performance, primarily by increasing muscle mass. Creatine is also being investigated as a treatment of neuromuscular diseases, where it may aid in neuroprotection and by improving the cellular bioenergetic state.
Purification Methods
Likely impurities are creatinine and other guanidino compounds. It crystallises from the minimum volume of boiling H2O as the monohydrate. The hydrate is also obtained by dissolving in H2O and adding Me2CO. Drying under vacuum over P2O5 or drying at 100o gives the anhydrous base. The anhydrous base can be obtained also by dissolving the hydrate in H2O, seeding with the anhydrous base and cooling in ice. A m of 258 -268o(dec) was reported. The picrate crystallises from 17 parts of H2O with m of 218-220o(dec). [King J Chem Soc 2377 1930, Hoffmann et al. J Am Chem Soc 58 1730 1936, Mendel & Hodgkin Acta Cryst 7 443 1954, Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 2750 1961, Beilstein 4 III 1170, 4 IV 2425.]
InChI:InChI=1/C4H9N3O2/c1-7(4(5)6)2-3(8)9/h2H2,1H3,(H4,5,6,8,9)
6020-87-7 Relevant articles
Process for the preparation of creatine water (by machine translation)
-
Paragraph 0014; 0041; 0042, (2016/10/07)
The invention discloses a preparation method of creatine monohydrate, and provides the preparation method of the creatine monohydrate. The method comprises the following steps: step 1, carrying out a nucleophilic substitution reaction on a glycolonitrile aqueous solution and a methylamine aqueous solution for 1 to 6 hours at a temperature of 10 DEG C to 40 DEG C so as to generate methylamino acetonitrile; step 2, carrying out a hydrolysis reaction on the methylamino acetonitrile reaction liquid obtained in the step 1 for 2 to 6 hours at the temperature of 60 DEG C to 80 DEG C and in the presence of sodium hydroxide so as to obtain a sodium sarcosinate aqueous solution; step 3, regulating pH to 9 to 12 and carrying out a condensation reaction on the sodium sarcosinate aqueous solution and the cyanamide for 1 to 6 hours at the temperature of 50 DEG C to 90 DEG C so as to obtain the creatine monohydrate. The method is moderate in reaction condition, less in byproduct, high in yield and suitable for industrial production. The structure of the creatine monohydrate is as shown in the specification.
Clean production method of mercaptan compound
-
Paragraph 0025; 0026, (2017/01/02)
The invention provides a clean production method of a mercaptan compound. There are two technological steps: Step 1, thiourea and halogenated hydrocarbon or active conjugated alkene react at 20-150 DEG C for 1-18 h, and after neutralization, S-alkylisothiourea is obtained; and Step 2, S-alkylisothiourea and aliphatic primary amine or secondary amine react at 20-180 DEG C for 1-24 h to obtain the mercaptan compound, and simultaneously, a substituted guanidino compound is coproduced. The mercaptan production technology has mild condition and high yield, hardly has discharge of ''three wastes (waste gas, waste water and industrial residue) '', and is a clean production method.
PROCESS FOR STRAIGHTENING KERATIN FIBRES WITH A HEATING MEANS AND DENATURING AGENTS
-
, (2010/03/02)
The invention relates to a process for straightening keratin fibres, comprising: (i) a step in which a straightening composition containing at least two denaturing agents is applied to the keratin fibres, (ii) a step in which the temperature of the keratin fibres is raised, using a heating means, to a temperature of between 110 and 250° C.
Preparation of substituted guanidine derivative
-
, (2008/06/13)
Substituted guanidine derivatives of the formula I, STR1 are prepared by reacting haloformamidinium salts of the formula II, STR2 where Hal can be Cl, F, Br and I, with primary or secondary amines of the formula III STR3 where the substituents R1 and R2 have the meanings explained in the description.
6020-87-7 Process route
-

- 107-97-1,25951-24-0
sarcosine

-
![2-{[amino(imino)methyl]sulfanyl}acetic acid](/upload/2023/1/daefc3f8-15c9-4527-954f-11bf3e0cdaad.png)
- 7404-50-4
2-{[amino(imino)methyl]sulfanyl}acetic acid

-

- 6020-87-7
creatine monohydrate powder
| Conditions | Yield |
|---|---|
|
at 60 ℃; for 3h;
|
80% |
-

- 420-04-2
CYANAMID

-

- 4316-73-8
sodium sarcosinate

-

- 6020-87-7
creatine monohydrate powder
| Conditions | Yield |
|---|---|
|
In water;
|
|
|
With water; at 20 - 80 ℃; for 1h; Temperature; Large scale;
|
6020-87-7 Upstream products
-
420-04-2

CYANAMID
-
4316-73-8

sodium sarcosinate
-
107-97-1

sarcosine
-
7404-50-4

2-{[amino(imino)methyl]sulfanyl}acetic acid
6020-87-7 Downstream products
-
60-27-5

creatinine
-
57-00-1

Creatinine
Relevant Products
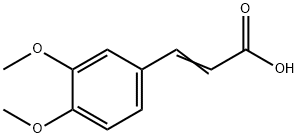
-
3,4-Dimethoxycinnamic acid
CAS:2316-26-9
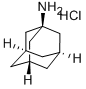
-
1-Adamantanamine hydrochloride
CAS:665-66-7