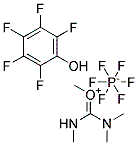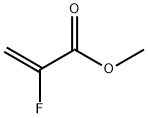
2343-89-7
- Product Name:2-Propenoic acid,2-fluoro-, methyl ester
- Molecular Formula:C4H5FO2
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 2343-89-7
Molecular Formula: C4H5FO2
2343-89-7 Properties
- Molecular Formula:C4H5FO2
- Molecular Weight:104.081
- Vapor Pressure:103mmHg at 25°C
- Refractive Index:1.39
- Boiling Point:75.9 °C at 760 mmHg
- Flash Point:1.1 °C
- PSA:26.30000
- Density:1.057 g/cm3
- LogP:0.64260
2343-89-7 Usage
Chemical Properties
Clear colorless liquid
Uses
Methyl 2-fluoroacrylate is an important raw material and intermediate used in organic synthesis, pharmaceuticals and agrochemicals.
Synthesis Reference(s)
Synthetic Communications, 11, p. 901, 1981 DOI: 10.1080/00397918108065745
InChI:InChI=1/C4H5FO2/c1-3(5)4(6)7-2/h1H2,2H3
2343-89-7 Relevant articles
ONE STEP SYNTHESIS FOR ALKYL 2-FLUOROACRYLATES
-
Paragraph 0073-0078, (2021/05/21)
A process is provided that results in an alkyl haloacrylate that is produced by reaction of a dialkyl or diaryl halomalonate with an aldehyde, preferably formalin or paraformaldehyde, and a base catalyst to produce an intermediate that is not isolated and is heated to produce the alkyl haloacrylate. This synthesis can be one pot, meaning it reacts in the same vessel and/or reaction mixture and does not require isolation of the intermediate, and provides an improved yield. In particular, a process is provided that results in an alkyl 2-fluoroacrylate.
METHOD FOR PRODUCING alpha-FLUOROACRYLIC ACID
-
Paragraph 0289-0306; 0309-0312; 0315-0320; 0322-0325; 0328-0, (2021/09/03)
An object of the present invention is to provide a novel method for producing an α-fluoroacrylic acid ester compound. This problem is solved by a method for producing a compound represented by formula (1), wherein R1 and R2 are identical or different, and each represents an alkyl group or the like; and R3 is an alkyl group or the like, the method comprising step A of reacting a compound represented by formula (2) with R3—OH (3) and carbon monoxide in the presence of palladium, a double bond-containing compound (α), a diphosphine compound (β), and a base, to obtain the compound represented by formula (1) above.
SYNTHESIS OF METHYL 2-FLUOROACRYLATE
-
Paragraph 00103-00106, (2021/10/02)
Methods for the synthesis of methyl 2-fluoroacrylate (MFA) are provided. The methods include use of various hydrofluorination agents using a variety of starting materials and reaction schemes. The methyl 2-fluoroacrylate prepared by the methods described herein can further be used to prepare patiromer calcium sorbitex.
METHOD OF PRODUCING HALOGENATED ACRYLIC ACID ESTER
-
Paragraph 0076-0078, (2021/06/25)
PROBLEM TO BE SOLVED: To provide a method allowing production of a halogenated acrylic acid ester in a manner that is safe and convenient and is also suitable for industrial production, without using toxic ingredients. SOLUTION: A method of producing a compound represented by formula (4) includes reacting a compound represented by formula (1) with a compound represented by formula (2) in the presence of a base. [In the formulas, each symbol is as described in the specification.] SELECTED DRAWING: None COPYRIGHT: (C)2021,JPOandINPIT
2343-89-7 Process route
-

- 2365-93-7
methyl 3-bromo-2-fluoro-2-chloropropanoate

-

- 2343-89-7
methyl 2-fluoroprop-2-enoate
| Conditions | Yield |
|---|---|
|
With 10H-phenothiazine; sulfuric acid; zinc; In water; at 82 - 86 ℃; for 0.333333h;
|
85% |
|
With hydrogen sulfide; sodium N-nitroso-N-phenylhydroxylaminate; zinc; In water; at 100 ℃;
|
82% |
|
With zinc;
|
80% |
|
With sulfuric acid; zinc;
|
-

- 55900-27-1
methyl 2-fluoro-3-chloropropionate

-

- 2343-89-7
methyl 2-fluoroprop-2-enoate
| Conditions | Yield |
|---|---|
|
With 2,6-di-tert-butyl-4-methyl-phenol; sodium phosphate; In 1-methyl-pyrrolidin-2-one; at 150 ℃; under 112.511 - 225.023 Torr;
|
95% |
|
With dimethyl amine; In diethyl ether; benzene;
|
2343-89-7 Upstream products
-
2365-93-7

methyl 3-bromo-2-fluoro-2-chloropropanoate
-
399-86-0

methyl 3-bromo-2-fluoropropionate
-
55900-27-1

methyl 2-fluoro-3-chloropropionate
-
380-26-7

methyl α,β-dibromo-α-fluoropropionate
2343-89-7 Downstream products
-
99953-33-0

methyl 3-bromo-2,2-difluoropropanoate
-
1279669-60-1

methyl 1-benzyl-3-fluoropyrrolidine-3-carboxylate
-
1417912-31-2

(2R,4S,5R)-dimethyl 5-(4-chlorophenyl)-4-fluoropyrrolidine-2,4-dicarboxylate
-
1417912-32-3

(2R,4S,5R)-dimethyl 5-(3-chlorophenyl)-4-fluoropyrrolidine-2,4-dicarboxylate
Relevant Products
-
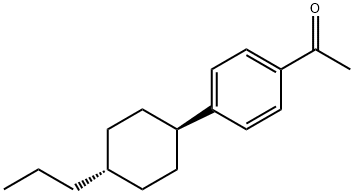
Ethanone,1-[4-(trans-4-propylcyclohexyl)phenyl]-
CAS:78531-61-0
-
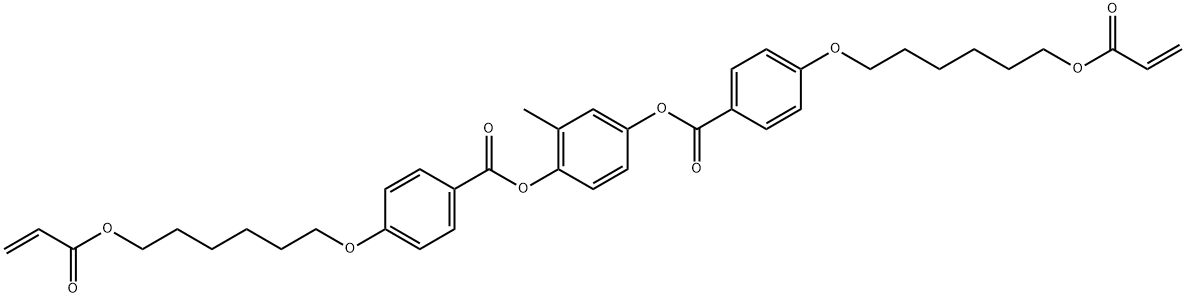
1,4-Bis-[4-(6-acryloyloxyhexyloxy)benzoyloxy]-2-methylbenzene
CAS:125248-71-7