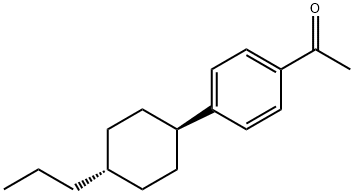
78531-61-0
- Product Name:Ethanone,1-[4-(trans-4-propylcyclohexyl)phenyl]-
- Molecular Formula:C17H24 O
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 78531-61-0
Molecular Formula: C17H24 O
78531-61-0 Properties
- Molecular Formula:C17H24 O
- Molecular Weight:244.377
- Boiling Point:363.3±21.0 °C(Predicted)
- PSA:17.07000
- Density:0.953±0.06 g/cm3(Predicted)
- LogP:4.96310
78531-61-0 Usage
Chemical Properties
White solid
Flammability and Explosibility
Notclassified
InChI:InChI=1/C17H24O/c1-3-4-14-5-7-16(8-6-14)17-11-9-15(10-12-17)13(2)18/h9-12,14,16H,3-8H2,1-2H3/t14-,16-
78531-61-0 Relevant articles
KETONES-NEMATOGENS WITH MODERATE NEGATIVE DIELECTRIC ANISOTROPY AND HIGH CLEARING POINTS.
Osman,Huynh-Ba
, p. 141 - 152 (2007/10/02)
Anisotropic ketones whose rigid cores contain phenyl and cyclohexyl units were synthesized. The carbonyl group was incorporated either in the terminal substituents or in the link between the core units. The mesomorphic behavior of these ketones is described and correlated with their chemical structure. The 1-(4-alkanoylphenyl)-4-trans-alkylcyclohexanes as well as the 1-(4-alkanoylphenyl)-4-trans-alkanoylcyclohexanes show nematic phases. In contrast to laterally substituted compounds, these ketones have clearing points which are higher than those of the corresponding hydrocarbons. Another advantage of these nematogens is the ease of synthesis.
SYNTHESIS AND SOME PROPERTIES OF NEMATIC COMPOUNDS CONTAINING THREE RING SYSTEMS.
Takatsu,Takeuchi,Sato
, p. 311 - 319 (2007/10/02)
Four series of nematic compounds were prepared and their transition temperatures and transition entropies measured. Their flow-aligned viscosities and birefringences were determined by extrapolation. 7 refs.
SYNTHESIS OF TRANS-4-ALKYL-1-PHENYLCYCLOHEXANES AND THEIR DERIVATIVES
Bezborodov, V. S.,Bubel', O. N.,Konovalov, V. A.,Ptashnikov, Yu. L.
, p. 1479 - 1483 (2007/10/02)
The alkylation of benzene by 1-alkanoyl-2-chlorocyclohexanes and 1-alkanoyl-1-cyclohexenes leads to the formation of cis- and trans-4-alkanoyl-1-phenylcyclohexanes, the yields of which increase with increase in the reaction temperature. trans-4-Alkyl-1-phenylcyclohexanes were obtained from 4-alkanoyl-1-phenylcyclohexanes and also from the products from the reaction of 4-alkylcyclohexanones with phenylmagnesium bromide.Some mesomorphous derivatives of these hydrocarbons were synthesized.
CONVENIENT SYNTHESIS OF 4-(TRANS-4 prime -N-ALKYLCYCLOHEXYL) BENZOIC ACIDS.
Szczucinski,Dabrowski
, p. 55 - 64 (2007/10/02)
A convenient method of obtaining 4-(trans-4 prime -n-alkylcyclohexyl) benzoic acids from alkanoyl chlorides, cyclohexane and benzene is described. Homologous series of above mentioned acids and their nitriles with alkyl tail 2 to 10 carbon atoms were prepared and temperatures of their phase transitions were determined.
78531-61-0 Process route
-

- 71-43-2,26181-88-4,54682-86-9,13967-78-7,174973-66-1
benzene

-
![1-[4-(4-Propyl-cyclohexyl)-phenyl]-ethanone](/upload/2023/1/99ebfa56-ee84-42e0-bab1-c49f5c009956.png)
- 78531-61-0
1-[4-(4-Propyl-cyclohexyl)-phenyl]-ethanone
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: AlCl3 / 45 °C
2: hydrazine hydrate / bis-(2-hydroxy-ethyl) ether / or in 1:1 mixture of triethanolamine and glycerol
3: AlCl3 / 1,2-dichloro-ethane / 0.5 h
With aluminium trichloride; hydrazine hydrate; In 1,2-dichloro-ethane; diethylene glycol;
|
|
|
Multi-step reaction with 5 steps
1: AlCl3 / 45 °C
2: NaBH4 / propan-1-ol / 4 h / 30 - 35 °C
3: p-toluenesulfonic acid / toluene / Heating
4: H2 / 10percent Pd/C / ethanol
5: AlCl3 / 1,2-dichloro-ethane / 0.5 h
With sodium tetrahydroborate; aluminium trichloride; hydrogen; toluene-4-sulfonic acid; palladium on activated charcoal; In propan-1-ol; ethanol; 1,2-dichloro-ethane; toluene;
|
|
|
Multi-step reaction with 3 steps
1: AlCl3 / 45 °C
2: hydrazine hydrate / bis-(2-hydroxy-ethyl) ether / or in 1:1 mixture of triethanolamine and glycerol
3: AlCl3 / 1,2-dichloro-ethane / 0.5 h
With aluminium trichloride; hydrazine hydrate; In 1,2-dichloro-ethane; diethylene glycol;
|
|
|
Multi-step reaction with 5 steps
1: AlCl3 / 45 °C
2: NaBH4 / propan-1-ol / 4 h / 30 - 35 °C
3: p-toluenesulfonic acid / toluene / Heating
4: H2 / 10percent Pd/C / ethanol
5: AlCl3 / 1,2-dichloro-ethane / 0.5 h
With sodium tetrahydroborate; aluminium trichloride; hydrogen; toluene-4-sulfonic acid; palladium on activated charcoal; In propan-1-ol; ethanol; 1,2-dichloro-ethane; toluene;
|
-

- 1655-03-4
1-cyclohex-1-enyl-propan-1-one

-
![1-[4-(4-Propyl-cyclohexyl)-phenyl]-ethanone](/upload/2023/1/99ebfa56-ee84-42e0-bab1-c49f5c009956.png)
- 78531-61-0
1-[4-(4-Propyl-cyclohexyl)-phenyl]-ethanone
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: AlCl3 / 45 °C
2: hydrazine hydrate / bis-(2-hydroxy-ethyl) ether / or in 1:1 mixture of triethanolamine and glycerol
3: AlCl3 / 1,2-dichloro-ethane / 0.5 h
With aluminium trichloride; hydrazine hydrate; In 1,2-dichloro-ethane; diethylene glycol;
|
|
|
Multi-step reaction with 5 steps
1: AlCl3 / 45 °C
2: NaBH4 / propan-1-ol / 4 h / 30 - 35 °C
3: p-toluenesulfonic acid / toluene / Heating
4: H2 / 10percent Pd/C / ethanol
5: AlCl3 / 1,2-dichloro-ethane / 0.5 h
With sodium tetrahydroborate; aluminium trichloride; hydrogen; toluene-4-sulfonic acid; palladium on activated charcoal; In propan-1-ol; ethanol; 1,2-dichloro-ethane; toluene;
|
78531-61-0 Upstream products
-
74-83-9

methyl bromide
-
61203-99-4

trans-4-propyl-(4'-cyanophenyl)cyclohexane
-
61203-94-9

trans-4-propyl-1-phenylcyclohexane
-
75-36-5

acetyl chloride
78531-61-0 Downstream products
-
65355-29-5

4-(trans-4'-propylcyclohexyl)-benzoic acid
-
87581-96-2

N-[4-(4-Propyl-cyclohexyl)-phenyl]-acetamide
-
61203-99-4

trans-4-propyl-(4'-cyanophenyl)cyclohexane
-
73163-49-2

4-(trans-4-propylcyclohexyl)benzamide
Relevant Products
-
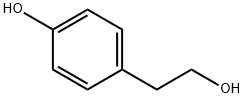
4-Hydroxyphenethyl alcoholCAS NO.: 501-94-0
CAS:501-94-0
-
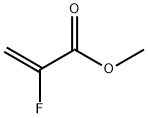
2-Propenoic acid,2-fluoro-, methyl ester
CAS:2343-89-7