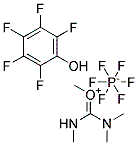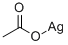
7377-03-9
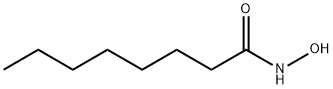
- Product Name:Octanamide, N-hydroxy-
- Molecular Formula:C8H17NO2
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 7377-03-9
Molecular Formula: C8H17NO2
7377-03-9 Properties
- Molecular Formula:C8H17NO2
- Molecular Weight:159.228
- Melting Point:78 °C
- Refractive Index:1.452
- PKA:9.56±0.20(Predicted)
- PSA:49.33000
- Density:0.97 g/cm3
- LogP:2.24320
7377-03-9 Usage
Uses
Octanohydroxamic Acid is used in preparation of Caprylohydroxamic Acid.
Uses
Octanohydroxamic Acid is used in preparation of Caprylohydroxamic Acid.
InChI:InChI=1/C8H17NO2/c1-2-3-4-5-6-7-8(10)9-11/h11H,2-7H2,1H3,(H,9,10)
7377-03-9 Relevant articles
Influence of octanohydroxamic acid on the association behavior of cationic surfactants: Hydrolytic cleavage of phosphate ester
Satnami, Manmohan L.,Dewangan, Hitesh K.,Kandpal, Neha,Nagwanshi, Rekha,Ghosh, Kallol K.
, p. 805 - 814 (2016)
The surface properties and mixed micellization behavior of cetyltrimethylammonium bromide (CTAB), tetradecyltrimethylammonium bromide (TTAB) and dodecyltrimethylammonium bromide (DTAB) with octanohydroxamic acid (OHA) have been investigated by means of conductivity and surface tension measurements in aqueous solution and borate buffer at 300 K. The critical micelle concentration (cmc), surface properties such as maximum surface access (Γmax), surface pressure at the cmc (Πcmc) and minimum surface area per molecule (Amin) has been determined. The standard Gibbs free energy of micellization (ΔGm0), standard Gibbs free energy of adsorption (ΔGad0), and standard Gibbs free energy of micellization per alkyl chain (ΔGm,tail0) of cationic surfactant with OHA have been evaluated. The fluorescence quenching technique was used to estimate the aggregation number (Nagg) and packing parameter (P) for determining the structural feature of cationic surfactants in the presence of octanohydroxamic acid. The hydrolytic reaction of paraoxon with octanohydroxamic acid was studied under a cationic micellar system by using OHA- at 9.2 pH and 300 K. The variations of surface properties from aqueous medium to the reaction condition have also been discussed. Pseudophase model (PPM) has been fitted for the quantitative treatment of the data.
Repurposing the 3-Isocyanobutanoic Acid Adenylation Enzyme SfaB for Versatile Amidation and Thioesterification
Zhu, Mengyi,Wang, Lijuan,He, Jing
supporting information, p. 2030 - 2035 (2020/11/30)
Genome mining of microbial natural products enables chemists not only to discover the bioactive molecules with novel skeletons, but also to identify the enzymes that catalyze diverse chemical reactions. Exploring the substrate promiscuity and catalytic mechanism of those biosynthetic enzymes facilitates the development of potential biocatalysts. SfaB is an acyl adenylate-forming enzyme that adenylates a unique building block, 3-isocyanobutanoic acid, in the biosynthetic pathway of the diisonitrile natural product SF2768 produced by Streptomyces thioluteus, and this AMP-ligase was demonstrated to accept a broad range of short-chain fatty acids (SCFAs). Herein, we repurpose SfaB to catalyze amidation or thioesterification between those SCFAs and various amine or thiol nucleophiles, thereby providing an alternative enzymatic approach to prepare the corresponding amides and thioesters in vitro.
Thioether-Directed NiH-Catalyzed Remote γ-C(sp3)-H Hydroamidation of Alkenes by 1,4,2-Dioxazol-5-ones
Chen, Qishu,Du, Bingnan,Ouyang, Yuxin,Yu, Wing-Yiu
supporting information, p. 14962 - 14968 (2021/09/29)
A NiH-catalyzed thioether-directed cyclometalation strategy is developed to enable remote methylene C-H bond amidation of unactivated alkenes. Due to the preference for five-membered nickelacycle formation, the chain-walking isomerization initiated by the NiH insertion to an alkene can be terminated at the γ-methylene site remote from the alkene moiety. By employing 2,9-dibutyl-1,10-phenanthroline as the ligand and dioxazolones as the reagent, the amidation occurs at the γ-C(sp3)-H bonds to afford the amide products in up to 90% yield (>40 examples) with remarkable regioselectivity (up to 24:1 rr).
Preparation process of capryloyl hydroxamic acid
-
Paragraph 0034-0042; 0046-0054, (2021/01/30)
The invention relates to the field of chemical synthesis, and particularly discloses a preparation process of capryloyl hydroxamic acid, which comprises the following steps: preparing ethyl n-caprylate, and preparing the capryloyl hydroxamic acid, wherein the ethyl n-caprylate is prepared by the following steps: mixing 1-1.5 kg of n-caprylic acid with 0.35-0.7 kg of ethanol, adding solid superacid, performing heating reflux for 5-7 hours, filtering to obtain precipitate and filtrate, and performing reduced pressure distillation on the filtrate to obtain the ethyl n-caprylate, and the obtainedprecipitate is washed and roasted to obtain the solid superacid, and the solid superacid is recycled to the preparation step of the ethyl n-caprylate for use. By using the solid superacid catalyst, the environmental friendliness of the esterification reaction in the n-capryloyl hydroxamic acid preparation process is improved.
METHOD FOR PREPARING N-HYDROXYALKANAMIDE WITH HIGH PURITY
-
Paragraph 0062-0076; 0081-0082, (2021/05/04)
The present application relates to a method for preparing N-hydroxyalkaneamide, comprising the steps of: (a) preparing a reaction solution by adding an organic solvent and a surfactant to an aqueous hydroxylamine solution; and (b) adding a carboxylic acid ester to the reaction solution to obtain N-hydroxyalkanamide having 6 to 10 carbon atoms, wherein the organic solvent is insoluble in water.
7377-03-9 Process route
-

- 106-32-1
octanoic acid ethyl ester

-

- 7377-03-9
caprylohydroxamic acid
| Conditions | Yield |
|---|---|
|
octanoic acid ethyl ester; With sodium ethanolate; sodium carbonate; In ethanol; at 40 ℃; for 2.5h;
With hydroxylamine; In ethanol; water;
|
95.7% |
|
With sodium sulfide; hydroxylamine hydrochloride; sodium hydroxide; In ethanol; at 20 - 45 ℃; for 3h; Temperature; Concentration;
|
93.1% |
|
With hydroxylamine hydrochloride; potassium hydroxide; In ethanol; water; at 5 - 55 ℃; for 3h; Concentration; Temperature;
|
91.1% |
|
With hydroxylamine; sodium acetate; sodium carbonate; In ethanol; water; at 27 ℃; for 2.5h; Temperature;
|
76.6% |
|
With hydroxylamine hydrochloride; sodium ethanolate;
|
|
|
With potassium hydroxide; hydroxylamine hydrochloride; In methanol; for 5h; temp. < 30 deg C;
|
|
|
With hydroxylamine hydrochloride; sodium hydroxide; In methanol; at 0 - 5 ℃;
|
|
|
With hydroxylamine hydrochloride;
|
-

- 111-11-5
methyl octanate

-

- 7377-03-9
caprylohydroxamic acid
| Conditions | Yield |
|---|---|
|
With hydroxylamine hydrochloride; sodium hydroxide; In methanol; water; at 5 - 40 ℃; for 5h; Concentration; Temperature; Large scale;
|
94.7% |
|
With hydroxylamine hydrochloride; sodium hydroxide; at 30 ℃; for 6h; pH=13;
|
92.3% |
|
With hydroxylamine hydrochloride; triethylamine; In water; at 0 - 50 ℃; for 14.5h; Temperature;
|
85% |
|
With hydroxylamine hydrochloride; calcium oxide; In methanol; at 10 - 50 ℃; for 2.5h; Temperature; Solvent; Autoclave;
|
83% |
|
With hydroxyammonium sulfate; calcium oxide; In methanol; at 50 ℃; for 2h; Solvent; Temperature;
|
80.3% |
|
methyl octanate; With hydroxylamine hydrochloride; In methanol; water; at 20 ℃; for 0.666667h;
With dmap; sodium hydroxide; In methanol; water; at 5 - 20 ℃; for 1h; pH=5; Solvent; Temperature; Reagent/catalyst; pH-value;
|
7377-03-9 Upstream products
-
124-07-2

Octanoic acid
-
106-32-1

octanoic acid ethyl ester
-
629-37-8

1-nitrooctane
-
111-64-8

n-octanoic acid chloride
7377-03-9 Downstream products
-
4747-81-3

N-heptylisocyanate
-
125640-84-8

N-benzyloxycarbonylheptylamine
-
14609-74-6

p-nitrophenolate
-
342371-84-0

9-deoxo-6-deoxy-6,9-epoxy-9,9a-didehydro-9a-aza-homoerythromycin A
Relevant Products
-
Aceticacid, silver(1+) salt (1:1)
CAS:563-63-3
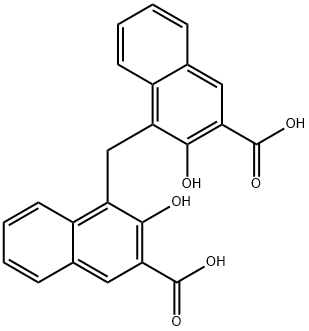
-
Pamoic acid
CAS:130-85-8