Product Details;
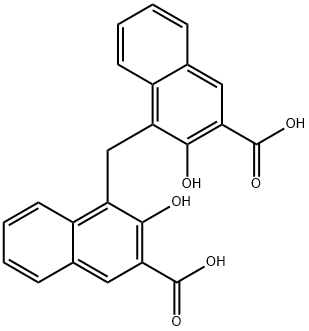
CasNo: 130-85-8
Molecular Formula: C23H16O6
Appearance: Fine Yellow Powder
130-85-8 Properties
- Molecular Formula:C23H16O6
- Molecular Weight:388.376
- Appearance/Colour:Fine Yellow Powder
- Vapor Pressure:2.13E-17mmHg at 25°C
- Melting Point:≥300ºC (dec.)
- Refractive Index:1.4593 (estimate)
- Boiling Point:642.7 ºC at 760 mmHg
- PKA:2.67±0.30(Predicted)
- Flash Point:356.5ºC
- PSA:115.06000
- Density:1.499 g/cm3
- LogP:4.39140
130-85-8 Usage
Chemical Properties
Pamoic acid is yellow to yellow-green crystalline powder
Uses
Agonist of the orphan G protein-coupled receptor GPR35: a potent activator of extracellular signal-regulated kinase and β-arrestin2 with antinociceptive activity. Used as an inhibitor in the real-time fluorescence enzymatic characterization study of specialized human DNA polymerases.
Uses
Pamoic acid is used in preparation of repository derivatives of medicinal agents, used in pharmacology to increase the solubility of a drug in water.
General Description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Safety Profile
Poison by intraperitoneal route. An eye irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
InChI:InChI=1/C23H16O6/c24-20-16(14-7-3-1-5-12(14)9-18(20)22(26)27)11-17-15-8-4-2-6-13(15)10-19(21(17)25)23(28)29/h1-10,24-25H,11H2,(H,26,27)(H,28,29)/p-2
130-85-8 Relevant articles
Synthesis of dihomocalix[4]naphthalenes: First members of a new class of [1.2.1.2](1,3)naphthalenophanes
Georghiou, Paris E.,Li, Zhaopeng,Ashram, Muhammad,Miller, David O.
, p. 3865 - 3869 (1996)
-
TREATMENT OF DISORDERS ASSOCIATED WITH G PROTEIN-COUPLED RECEPTOR 35 (GPR35)
-
Page/Page column 18, (2011/02/24)
Compounds are provided having agonistic activity against G protein- coupled receptor 35 (GPR35). The compounds are useful for providing antinociception, providing neuroprotection in case of stroke or ischemia, or treating gastric inflammation.
ALKYLENEBISNAPHTOL DERIVATIVES AND CHARGE CONTROL AGENT COMPRISING THE SAME
-
Page 9-10, (2008/06/13)
The present invention provides an alkylenebisnaphthol derivative represented by formula [I]: and a salt thereof. The compound of the present invention has an excellent triboelectric charge property and useful as charge control agent for electrophotographic toners.
Crystalline cepham acid addition salts and processes for their preparation
-
, (2008/06/13)
Novel crystalline cephem acid addition salts and processes for their preparation Compounds of the formula I STR1 in which n is equal to 1 or 2 and m is 0.4-2.6, and where X is the anion of a carboxylic acid, have antibacterial activity.
130-85-8 Process route
-

- 50-00-0,30525-89-4,61233-19-0
formaldehyd

-

- 92-70-6
3-Hydroxy-2-naphthoic acid

-

- 130-85-8
Pamoic acid
| Conditions | Yield |
|---|---|
|
With sulfuric acid; In acetic acid; for 10h; Heating;
|
95% |
|
With sodium hydroxide;
|
|
|
With sulfuric acid; acetic acid;
|
|
|
With sodium hydroxide;
|
-

-
9-(3-Carboxy-2-hydroxy-naphthalen-1-ylmethyl)-2,4-dioxo-4H-1,3-dioxa-2λ5-phospha-anthracen-2-ol anion

-

- 130-85-8
Pamoic acid
| Conditions | Yield |
|---|---|
|
With water; at 40 ℃; Rate constant; var. pHs;
|
130-85-8 Upstream products
-
50-00-0

formaldehyd
-
64-19-7

acetic acid
-
92-70-6

3-Hydroxy-2-naphthoic acid
130-85-8 Downstream products
-
27757-79-5

3-hydroxy-4-phenylazo-[2]naphthoic acid
-
49609-90-7

bis(methyl-2-methoxy-3-naphthoyl)methane
-
217327-71-4

C51H40O6
-
68902-24-9

1-methyl-2-hydroxy-3-naphthoic acid
Relevant Products
-
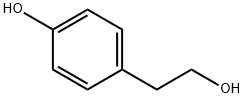
4-Hydroxyphenethyl alcoholCAS NO.: 501-94-0
CAS:501-94-0
-
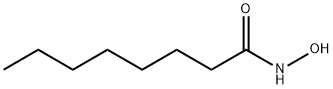
Octanamide, N-hydroxy-
CAS:7377-03-9