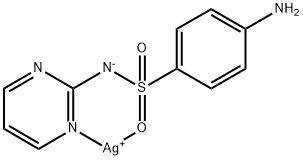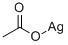
563-63-3
- Product Name:Aceticacid, silver(1+) salt (1:1)
- Molecular Formula:C2H3AgO2
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 563-63-3
Molecular Formula: C2H3AgO2
Appearance: Off-white/brown crystalline powder
563-63-3 Properties
- Molecular Formula:C2H3AgO2
- Molecular Weight:166.913
- Appearance/Colour:Off-white/brown crystalline powder
- Vapor Pressure:13.9mmHg at 25°C
- Melting Point:decomposes [STR93]
- Boiling Point:117.1°C at 760 mmHg
- Flash Point:40°C
- PSA:26.30000
- Density:3.25 g/cm3
- LogP:0.01380
563-63-3 Usage
Description
Silver acetate (C2H3AgO2) is a photosensitive, white, crystalline solid which is widely used in the laboratory. As a source of silver ions lacking an oxidizing anion, it is a useful reagent for direct ortho-arylation, and for conversion of organohalogen compounds into alcohols, etc. It also serves as a catalyst to effectively catalyze the cycloaddition reactions of isocyanoacetates with a variety of olefins. It can be employed in the novel preparation of highly reflective, conductive silvered polymer films.Besides, it has applications in some antismoking drugs and in the health field, in which the products containing silver acetate have been applied in spray, and lozenges to deter smokers from smoking. When mixed with smoke, the silver acetate creates an unpleasant metallic taste in the smoker's mouth, thereby preventing them from smoking.
References
https://en.wikipedia.org/wiki/Silver_acetate https://www.alfa.com/zh-cn/catalog/011660/ http://www.sigmaaldrich.com/catalog/product/sigma/s1633?lang=en®ion=US
Description
Silver acetate is an organic compound with the empirical formula CH3COOAg (or AgC2H3O2). It is a photosensitive, white crystalline solid. It is a useful reagent in the laboratory as a water soluble source of silver lacking an oxidizing anion. It has been used in some antismoking drugs.
Chemical Properties
Off-White/Brown Crystalline Powder
Uses
Oxidizing agent for use in liquid ammonia: Kline, Kershner, Inorg. Chem. 5, 932 (1966).
Uses
In the health field, silver acetate-containing products have been used in gum, spray, and lozenges to deter smokers from smoking. The silver in these products, when mixed with smoke, creates an unpleasant metallic taste in the smoker's mouth, thus deterring them from smoking. Lozenges containing 2.5 mg of silver acetate showed "modest efficacy" on 500 adult smokers tested over a three-month period. However, over a period of 12 months, prevention failed. In 1974, silver acetate was first introduced in Europe as an over-thecounter smoking-deterrent lozenge (Repaton) and then three years later as a chewing gum (Tabmint).
Uses
It is a reagent in the laboratory as a source of silver ions lacking an oxidizing anion. It is a reagent for direct ortho-arylation, and for conversion of organohalogen compounds into alcohols. Woodward cis-hydroxylation reaction employs silver acetate and iodine for selective conversin of alkenes into cis-diols. Silver acetate is the more preferred reagent for facile carbonylation of primary and secondary amines. It is also employed in the preparation of highly reflective, conductive silvered polymer films.
Reactions
3 – 1 - Carbonylation Silver acetate, when combined with carbon monoxide (CO), can induce the carbonylation of primary and secondary amines. Other silver salts can be used but the acetate gives the best yield. 2 R2NH + 2 AgOAc + CO → [R2N]2CO + 2 HOAc + 2 Ag 3 – 2 - Hydrogenation Silver acetate in a solution of pyridine absorbs hydrogen and is reduced to metallic silver. 3 – 3 - Direct ortho - arylation Silver acetate is a useful reagent for direct ortho-arylation (to install two adjacent substituents on an aromatic ring) for of benzylamines and N-methylbenzylamines. The reaction is palladiumcatalized and requires a slight excess of silver acetate.This reaction is shorter than previous ortho-arylation methods.
Brand name
Smokerette;Tabmint.
World Health Organization (WHO)
Silver acetate has been used as a disinfectant and as an antismoking aid. It was refused registration in Cyprus on the grounds that prolonged use of silver salts can cause permanent argyria and that no well-controlled trials have been performed to establish the safety and efficacy of the preparation. It remains registered as an aid to stopping smoking in Canada and the United States.
General Description
White crystalline plates. Light sensitive. Density 3.26 g / cm3.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Silver acetate is freely soluble in dilute nitric acid [Merck]. Can serve as an oxidizing agent.
Hazard
Toxic material.
Health Hazard
Inhalation of dust irritates nose and throat. Contact with eyes or skin causes irritation. If continued for a long period, ingestion or inhalation of silver compounds can cause permanent discoloration of skin (argyria).
Safety
The LD50 of silver acetate in mice is 36.7 mg/kg. Low doses of silver acetate in mice produced hyper-excitability, ataxia, central nervous system depression, labored breathing, and even death. The U.S. FDA recommends that silver acetate intake be limited to 756 mg over a short period of time; excessive intake may cause argyria.
Synthesis
The silver acetate salt can be synthesized by the reaction of acetic acid and silver carbonate at 45 – 60 °C. After allowing cooling to room temperature, the solid product precipitates. 2 CH3CO2H + Ag2CO3 → 2 AgO2CCH3 + H2O + CO2 It can also be precipitated from concentrated aqueous solutions of silver nitrate by treatment with a solution of sodium acetate. The structure of silver acetate consists of 8-membered Ag2O4C2 rings formed by a pair of acetate ligands bridging a pair of silver centres.
Purification Methods
Shake it with acetic acid for three days, and the process is repeated with fresh acid. The solid is then dried in a vacuum oven at 40o for 48hours. It has also been recrystallised from water containing a trace of acetic acid, and dried in air. Store it in the dark. [Beilstein 2 IV 112.]
InChI:InChI=1/C2H4O2.Ag/c1-2(3)4;/h1H3,(H,3,4);/q;+1/p-1
563-63-3 Relevant articles
Reactive silver inks for patterning high-conductivity features at mild temperatures
Walker, S. Brett,Lewis, Jennifer A.
, p. 1419 - 1421 (2012)
Reactive silver inks for printing highly conductive features (>10 4 S/cm) at room temperature have been created. These inks are stable, particle-free, and suitable for a wide range of patterning techniques. Upon annealing at 90 °C, the printed electrodes exhibit an electrical conductivity equivalent to that of bulk silver.
Study of thermal decomposition of silver acetate
Logvinenko,Polunina,Mikhailov,Mikhailov,Bokhonov
, p. 813 - 816 (2007)
Thermal decomposition of silver acetate was studied (TG, DSC, mass-spectrometry, X-ray analysis, electron microscopy). Non-isothermal thermogravimetric data (obtained at two different rates of linear heating) were used for kinetic studies. Kinetic paramet
The oxidation of uranium(IV) acetate by silver acetate in liquid ammonia
Kline, Robert J.,Kershner, Carl J.
, p. 932 - 934 (1966)
-
Free Amino Group-Directed γ-C(sp3)-H Arylation of α-Amino Esters with Diaryliodonium Triflates by Palladium Catalysis
Pramanick, Pranab K.,Zhou, Zhibing,Hou, Zhen-Lin,Yao, Bo
, (2019)
Free amino group-directed C(sp3)-H functionalization of aliphatic amines is a fundamental challenge in synthetic organic chemistry. Also, the NH2-directed C(sp3)-H functionalization of α-amino acids and their derivatives remains barely explored. With palladium as the catalyst and Ag2O as the additive, we developed the first NH2-directed γ-C(sp3)-H arylation of α-amino esters with diaryliodonium triflates for the construction of synthetically useful γ-aryl-α-amino esters, and the result of the KIE study suggested that the catalytic reaction involved an irreversible C-H cleavage as the rate-determining step.
New antitumour active platinum compounds containing carboxylate ligands in trans geometry: Synthesis, crystal structure and biological activity
Van Zutphen, Steven,Pantoja, Elena,Soriano, Rosario,Soro, Carlos,Tooke, Duncan M.,Spek, Anthony L.,Den Dulk, Hans,Brouwer, Jaap,Reedijk, Jan
, p. 1020 - 1023 (2006)
New asymmetric trans-platinum(ii) complexes, composed of an isopropylamine, an azole and two carboxylate leaving groups, are presented. The crystal and molecular structures of one of the complexes has been determined and the cytotoxicity and reactivity with 5′-guanosine monophosphate is reported. The complexes show a reduced reactivity, but no decrease in cytotoxic activity compared to their chloro-counterparts. Furthermore the complexes largely overcome cisplatin resistance, they therefore present an interesting class of antitumour active trans-platinum complexes. The Royal Society of Chemistry 2006.
Impact of counterions on micelle formation and polymerization of 11-acryloyloxyundecyltrimethylammonium surfactants
Bilibin, A. Yu,Fetin, P. A.,Fetina, V. I.,Lezov, A. A.,Zorin, I. M.
, (2020/04/20)
New surfactants based on the cationic monomer 11-acryloyloxyundecyltrimethylammonium with counterions bromide, nitrate, acetate, camphorsulfonate, 4-toluenesulfonate, trifluoroacetate were obtained in this work. In most cases counterions change the critical micelle concentration by 2–3 times (being compared with bromide) except sample with acetate counterions (in this case critical micelle concentration increases by an order of magnitude). Substitution of bromide for hydrophobic counterions (toluenesulfonate, trifluoroacetate, camphorsulfonate) does not lead to expected transformation of spherical micelles to long wormlike micelles. The polymerization of such monomers occurs according to the microemulsion mechanism involving micelles of monomers.
MANUFACTURING METHOD OF FLUORINATED HYDROCARBON
-
Paragraph 0059, (2018/05/08)
PROBLEM TO BE SOLVED: To provide a method for industrially advantageously manufacturing fluorinated hydrocarbon (3). SOLUTION: There is provided a method for manufacturing fluorinated hydrocarbon represented by the formula (3), including contacting a secondary or tertiary ether compound represented by the formula (1) and acid fluoride represented by the formula (2) in the presence of a silver salt in a hydrocarbon solvent. R1 and R2 are each independently a C1 to 3 alkyl group, R1 and R2 may bind to form a ring structure, R3 is H, a methyl group or an ethyl group, R4 and R5 are each independently a methyl group or an ethyl group. SELECTED DRAWING: None COPYRIGHT: (C)2018,JPOandINPIT
Experimental and mechanistic analysis of the palladium-catalyzed oxidative C8-selective C-H homocoupling of quinoline N-oxides
Stephens, David E.,Lakey-Beitia, Johant,Chavez, Gabriel,Ilie, Carla,Arman, Hadi D.,Larionov, Oleg V.
supporting information, p. 9507 - 9510 (2015/06/08)
A novel site-selective palladium-catalyzed oxidative C8-H homocoupling reaction of quinoline N-oxides has been developed. The reaction affords substituted 8,8′-biquinolyl N,N′-dioxides that can be readily converted to a variety of functionalized 8,8′-biquinolyls. Mechanistic studies point to the crucial role of the oxidant and a non-innocent behavior of acetic acid as a solvent.
563-63-3 Process route
-

- 127-09-3
sodium acetate

-

-
silver nitrate

-

- 563-63-3
silver(I) acetate
| Conditions | Yield |
|---|---|
|
With acetic acid; In water; at 23 ℃;
|
91% |
|
With acetic acid; In water; addn. of satd. aq. soln. of sodium acetate to satd. aq. soln. of AgNO3 contg. few drops of acetic acid in the dark; filtration, washing the residue with water, methanol, hexane, drying in vac.;
|
90% |
|
In water; soln. of AgNO3 in water was added to soln. of NaCH3CO2 in water; filtered in air, washed with water, EtOH, Et2O, dried in vacuo; elem. anal.;
|
64% |
|
In water; byproducts: NaNO3; investigation of equilibrium at 25°C;;
|
|
|
In water; pptn.;; washing free from nitrate, filtration (clay), drying in vac. over P2O5 until weight constance;;
|
|
|
In water; pptn.;; washing several times with H2O;;
|
|
|
In water; pptn.;; washing, recrystn. from H2O, drying in vac. over H2SO4 until weight constance;;
|
|
|
In water; preparation of a photographic emulsion described;;
|
|
|
In water; pptn.;; washing, recrystn. from H2O, drying in vac. over H2SO4 until weight constance;;
|
|
|
In water; pptn.;; washing free from nitrate, filtration (clay), drying in vac. over P2O5 until weight constance;;
|
-

-
silver nitrate

-

- 64-19-7,77671-22-8
acetic acid

-

- 563-63-3
silver(I) acetate
| Conditions | Yield |
|---|---|
|
With NaOH; In not given; neutralising CH3COOH with NaOH, treatment with AgNO3;
|
|
|
silver nitrate; With sodium hydroxide;
acetic acid;
|
563-63-3 Upstream products
-
53897-55-5

2,4,6-trimethyl-[1,3,5]dioxathiane
-
849585-22-4

LACTIC ACID
-
7732-18-5

water
-
64-19-7

acetic acid
563-63-3 Downstream products
-
6619-10-9

1,2,3,4-tetra-O-acetyl-6-O-p-tolylsulfonyl-β-D-glucopyranose
-
110-86-1

pyridine
-
120613-73-2

2,4-dinitro-benzeneselenenic acid methyl ester
-
51238-15-4

4β-acetoxycholest-5-en-3β-ol
Relevant Products
-
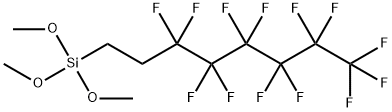
1H,1H,2H,2H-Perfluorooctyltrimethoxysilane
CAS:85857-16-5
-
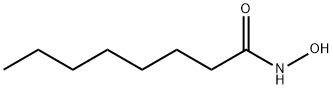
Octanamide, N-hydroxy-
CAS:7377-03-9