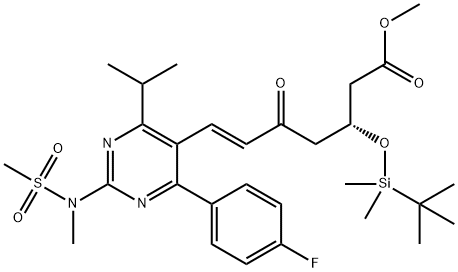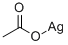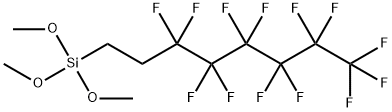
85857-16-5
- Product Name:1H,1H,2H,2H-Perfluorooctyltrimethoxysilane
- Molecular Formula:C11H13F13O3Si
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 85857-16-5
Molecular Formula: C11H13F13O3Si
85857-16-5 Properties
- Molecular Formula:C11H13F13O3Si
- Molecular Weight:468.287
- Vapor Pressure:0.197mmHg at 25°C
- Melting Point:<0 °C
- Refractive Index:1.335
- Boiling Point:217.3 °C at 760 mmHg
- Flash Point:85.2 °C
- PSA:27.69000
- Density:1.393 g/cm3
- LogP:4.99340
85857-16-5 Usage
Chemical Properties
liquid
Flammability and Explosibility
Nonflammable
InChI:InChI=1/C11H13F13O3Si/c1-25-28(26-2,27-3)5-4-6(12,13)7(14,15)8(16,17)9(18,19)10(20,21)11(22,23)24/h4-5H2,1-3H3
85857-16-5 Relevant articles
Method for synthesizing flurosilicon compound by using continuous flow microreactor
-
Paragraph 0045; 0046, (2019/04/10)
The invention belongs to the technical field of a fluorine-containing material, and particularly relates to a method for synthesizing a flurosilicon compound by using a continuous flow microreactor. The synthesis method is characterized in that the continuous flow microreactor is used as a reaction device; the method comprises the following steps of (1) flowing fluoroolefin, alkoxy silane and catalysts dissolved into organic solvents into a mixed reaction module M with the preset temperature T for mixed reaction respectively through metering pumps P1, P2 and P3 via a material passage A, a material passage B and a material passage C; (2) after the mixed reaction is completed, flowing reaction liquid flows out of an outlet D; performing post treatment by flow-out reaction liquid to obtain the flurosilicon compound. The synthesis method provided by the invention has the advantages of scientificalness, reasonableness, simplicity and easy implementation. The problems of serious heat release, difficult operation control, rigorous equipment requirement, low yield, long reaction time and the like in the existing chlorsilane silicon and hydrogen addition can be solved.
PERFLUORO-ALKYL MODIFIED ORGANIC SILANE
-
Paragraph 0095; 0096, (2017/05/27)
The present invention relates to a method for preparing perfluoroalkyl modified organic silane, and the method for preparing perfluoroalkyl modified organic silane comprises a step for adding a pyrolytic radical initiator and a tin-based organic metal compound to a mixture of a perfluoroalkyl iodide compound and an organic silane having a double bond. The method for preparing perfluoroalkyl modified organic silane suppresses an additive reaction of the pyrolytic radical initiator and smoothens the reduction of iodine, thereby forming perfluoroalkylalkoxy silane at high efficiency and high purity, causes no chlorine gas, which is a toxic gas, thereby making less environmental loading, such as gas purification facilities, and does not use an expensive metal catalyst and hydrogen gas, thereby preparing perfluoroalkyl modified organic silane easily and economically.COPYRIGHT KIPO 2016
Syntheses and Reactions of Metal Organics. XVIII. Syntheses of (1H,1H,2H,2H-Polyfluoroalkyl)trimethoxysilanes and Surface Modification of Glass Plate
Yoshino, Norio,Yamamoto, Yasushi,Hamano, Katsumi,Kawase, Tokuzo
, p. 1754 - 1758 (2007/10/02)
Four silane coupling agents, (1H,1H,2H,2H-polyfluoroalkyl)trimethoxysilanes ((1H,1H,2H,2H-henicosafluorododecyl)trimethoxysilane, C10F21C2H4Si(OCH3)3, (1H,1H,2H,2H-heptadecafluorodecyl)trimethoxysilane, C58F17C2H4Si(OCH3)3, (1H,1H,2H,2H-tridecafluorooctyl)trimethoxysilane, C6F13C2H4Si(OCH3)3, and (1H,1H,2H,2H-nonafluorohexyl)trimethoxysilane, C4F9C2H4Si(OCH3)3), were prepared by the hydrosilylation of trichlorosilane with the corresponding 1H,1H,2H,-polyfluoro-1-alkene in the presence of hydrogen hexachloroplatinate-(IV), followed by reaction with sodium methoxide.The surface modification of glass plate was attempted using these products.From measurements of the contact angles θ (deg) of water and oleic acid against a modified glass plate surface, the coupling agents were found to have high modification ability.The modification produced a glass surface with high oxidation resistance.
85857-16-5 Process route
-

- 2487-90-3
trimethoxysilane

-

- 25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene

-

- 85857-16-5
(1H,1H,2H,2H-tridecafluorooctyl)trimethoxysilane
| Conditions | Yield |
|---|---|
|
With chloroplatinic acid; In isopropyl alcohol; at 25 ℃; for 0.133333h;
|
98.2% |
-

- 2768-02-7
(vinyl)trimethoxylsilane

-

- 355-43-1
n-perfluorohexyl iodide

-

- 85857-16-5
(1H,1H,2H,2H-tridecafluorooctyl)trimethoxysilane
| Conditions | Yield |
|---|---|
|
(vinyl)trimethoxylsilane; n-perfluorohexyl iodide; With 2,2'-azobis(isobutyronitrile); at 60 ℃; for 6h;
With tri-n-butyl-tin hydride; at 25 ℃; for 4h;
|
70% |
85857-16-5 Upstream products
-
124-41-4

sodium methylate
-
78560-45-9

(tridecafluoro-1,1,2,2-tetrahydrooctyl)trichlorosilane
-
25291-17-2

3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene
-
2768-02-7

(vinyl)trimethoxylsilane
Relevant Products
-
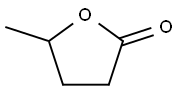
2(3H)-Furanone,dihydro-5-methyl-
CAS:108-29-2
-
Aceticacid, silver(1+) salt (1:1)
CAS:563-63-3