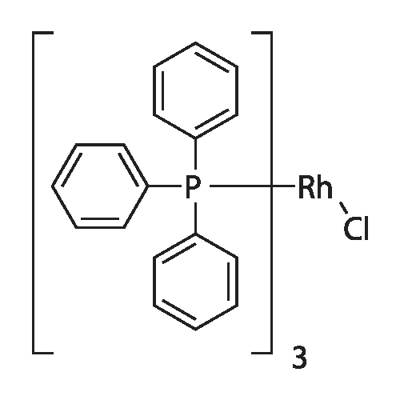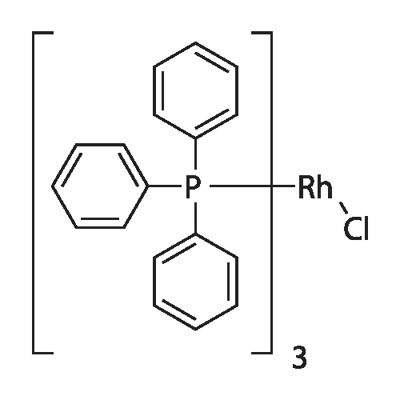
14694-95-2
- Product Name:Rhodium,chlorotris(triphenylphosphine)-, (SP-4-2)-
- Molecular Formula:C54H45ClP3Rh
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 14694-95-2
Molecular Formula: C54H45ClP3Rh
Appearance: magenta crystals
14694-95-2 Properties
- Molecular Formula:C54H45ClP3Rh
- Molecular Weight:925.231
- Appearance/Colour:magenta crystals
- Melting Point:245-250 °C (dec.)
- Boiling Point:>170 °C
- Flash Point:181.7oC
- PSA:40.77000
- Density:1.363; d 1.379
- LogP:11.02390
14694-95-2 Usage
Reactions
A homogeneous hydrogenation catalyst which operates under mild conditions. Catalyst for the decarbonylation of aldehydes. Catalyst for regio- and stereoselective allylic substitution reactions. Alkyne hydro-phosphorylation Heck-type reaction with α,β-unsaturated esters. Alkyne arylation Allylic alcohol-olefin coupling. Terminal alkenes from ketones. Rh-catalyzed isomerization of α-aryl propargyl alcohols to indanones. Reductive deprotection of silyl groups.
Chemical Properties
magenta crystals
Uses
suzuki reaction
Uses
Tris(triphenylphosphine)rhodiu is also known as Wilkinson's catalyst and is commonly used to catalyze the hydrogenation of alkenes. Tris(triphenylphosphine)rhodiu is also used in the catalytic hydroboration of alkenes with catecholborane and pinacolborane and the selective 1,4-reduction of α, β-unsaturated carbonyl compounds.
Uses
Homogeneous hydrogenation catalyst.
Flammability and Explosibility
Nonflammable
Purification Methods
It forms dark burgundy crystals from hot EtOH after refluxing for 30minutes. When the solution is heated for only 5minutes, orange crystals are formed. Heating the orange crystals in EtOH yields red crystals. Crystallisation from Me2CO gives the orange crystals. The two forms have similar IR spectra, but the X-ray diffraction patterns are slighly different. [Osborne et al. J Chem Soc (A) 1711 1966, Osborne & Wilkinson Inorg Synth X 67 1967, Bennett & Donaldson Inorg Chem 16 655 1977.] The solubilities are as follows: in CH2Cl2 ~2% (25o), in toluene 0.2% (25o), and less soluble in Me2CO, MeOH, BuOH and AcOH, but insoluble in pet ethers and cyclohexane. It reacts with donor solvents such as pyridine, DMSO and MeCN.
InChI:InChI=1/3C18H15P.ClH.Rh/c3*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;/h3*1-15H;1H;/q;;;;+1/p-1/r3C18H15P.ClRh/c3*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-2/h3*1-15H;
14694-95-2 Relevant articles
Oxygenation Studies. Part 7. Catalytic Dioxygenation of Cyclo-octa-1,5-diene at a Rhodium Centre
Read, Gordon,Urgelles, Miguel
, p. 1591 - 1596 (1985)
Studies on the oxygenation of cyclo-octa-1,5-diene by molecular oxygen using as catalysts (1), and related dioxygen complexes in the presence of excess PPh3 are reported.A regioselective homoco-oxygenation of the diene is found to occur with (1) to give cyclo-octane-1,4-dione, in competition with some homoco-oxygenation of PPh3. 18O-Labelling experiments establish that both oxygens in a molecule of (1) are transferred to a molecule of the diene and initial rate measurements indicate that the slow step in the catalytic cycle follows displacement of one phosphine ligand in (1) by the diene.An overall mechanistic cycle, involving a sequence of five-, seven-, and four-membered metallacyclic intermediates, is suggested.
Synthesis of a square-planar rhodium alkylidene N-heterocyclic carbene complex and its reactivity toward alkenes
Palacios, Laura,Miao, Xiaowei,Di Giuseppe, Andrea,Pascal, Simon,Cunchillos, Carmen,Castarlenas, Ricardo,Perez-Torrente, Jesus J.,Lahoz, Fernando J.,Dixneuf, Pierre H.,Oro, Luis A.
, p. 5208 - 5213 (2011)
The first rhodium alkylidene square-planar complex stabilized by an N-heterocyclic carbene ligand, RhCl(=CHPh)(IPr)PPh3 (2; IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-carbene), has been prepared by reaction of RhCl(IPr)(PPh3)2 (1) with phenyldiazomethane and its dynamic behavior in solution studied. Treatment of 2 with alkenes results in the formation of the η2-olefin complexes RhCl(η2- CH2=CHR)(IPr)PPh3 (3, R = H; 4, R = Ph; 5, R = OEt) and new olefins arising from the coupling of the alkylidene with the alkenes, likely via a metallacyclobutane intermediate.
Ethylene hydroformylation in imidazolium-based ionic liquids catalyzed by rhodium-phosphine complexes
Diao, Yanyan,Li, Jing,Wang, Ling,Yang, Pu,Yan, Ruiyi,Jiang, Li,Zhang, Heng,Zhang, Suojiang
, p. 54 - 62 (2013)
In this research, the catalytic activity of a rhodium-based (Rh) catalyst with imidazolium-based ionic liquids (IBILs) as solvents for ethylene hydroformylation was studied. The structures of IBILs had an important influence on the activity and stability of the Rh catalyst. The IBILs with longer cation side chains, which were the strong steric hindrances around the Rh catalyst, were more unfavorable for the catalytic activity. The turnover frequency (TOF) of the Rh catalyst was 10627 h-1 when [Bmim][BF4] was used as solvent. The activity of the Rh complexes in the ionic liquid is better than they do in toluene. We used electrospray ionization mass spectrometry to characterize the catalyst after the reaction and found that [Bmim]+ acts as a ligand of the Rh catalyst to form a new active catalytic site [Rh(CO)(PPh3)2(Bmim)(BF4)]+ through the coordination of the Rh atom with the imidazole-2-C group of [Bmim][BF 4], and it was essential for the stabilization of the Rh catalyst and prevented the formation of low-active Rh clusters. In addition, the catalyst recycling test showed that the Rh catalyst could be reused with [Bmim][BF 4] as solvent without obvious loss of catalytic activity.
Hydrogenation of acrylonitrile-butadiene rubber latex using in situ synthesized RhCl(PPh3)3 catalyst
Liu, Yin,Wei, Zhenli,Pan, Qinmin,Rempel, Garry L.
, p. 62 - 68 (2013)
Catalytic hydrogenation of acrylonitrile-butadiene rubber (NBR) latex was achieved by using in situ synthesized RhCl(PPh3)3 catalyst. Water-soluble rhodium salt (RhCl3) was used as the catalyst precursor which was reacted in situ with triphenylphosphine (PPh3) to form RhCl(PPh3)3. Compared with using solid RhCl(PPh3)3, hydrogenation of NBR latex using the in situ RhCl(PPh3)3 showed a faster hydrogenation reaction. Based on the retention of catalyst in the polymer, it was revealed that the efficiency of in situ synthesis and the diffusion of the catalyst from the aqueous phase into the polymer particles were crucial in achieving the required high degree of hydrogenation. As a result, it was discovered that by introducing a small amount of alcohol (e.g. RhCl3 = 0.52 mmol/L, PPh3 = 18 mmol/L, ethanol/total NBR latex = 1/10 volume ratio) to the feed latex, the in situ synthesized catalyst could be used in the hydrogenation reaction very efficiently.
Synthesis of Ammonium Ions and Nitrosylation Reactions using Nitrosyl Chloride and Alkyl Nitrites
Khan, M. Ishaque,Agarwala, U. C.
, p. 1139 - 1142 (1989)
Nitrosyl chloride (NOCl) reacts with RuCl3*xH2O in the presence of PPh3 in different alcohols leading to the formation of ammonium ions and under mild experimental conditions, through reductive deoxygenation.Some parameters affecting th
A ternary Rh complex catalyst highly active and stable in the hydrogenation of acrylonitrile-butadiene rubber
Cao, Peng,Wu, Meng,Zou, Rui,Zhang, Liqun,Yue, Dongmei
, p. 1583 - 1586 (2015)
An Rh-based complex, T-Rh-PPh3, was developed through a facile one-step process. The T-Rh-PPh3 exhibited high activity and air stability in the hydrogenation of acrylonitrile-butadiene rubber. The enhanced air stability can be ascribed to the phenolic hydroxyl structures in tannin.
Synthesis of Silicon and Germanium-Containing Heterosumanenes via Rhodium-Catalyzed Cyclodehydrogenation of Silicon/Germanium-Hydrogen and Carbon-Hydrogen Bonds
Zhou, Dandan,Gao, Ya,Liu, Bingxin,Tan, Qitao,Xu, Bin
, p. 4628 - 4631 (2017)
A three-step synthesis of C3-symmetric trisilasumanene and trigermasumanene, heteroanalogues of the π-bowl sumanene, was achieved using a threefold rhodium-catalyzed cyclodehydrogenation of Si/Ge-H and C-H bonds as the key step. Trigermasumanene was proven to adopt a planar geometry by single crystal X-ray diffraction for the first time. The optical properties were also investigated by UV-vis and fluorescence spectroscopy.
The Structure of Crystalline trans-Dichlorobis(triphenylphosphine)rhodium(II), a Square Planar Rhodium(II) Monomer: Isolation of the Proposed Paramagnetic Impurity in Wilkinson's Catalyst
Ogle, Craig A.,Masterman, T. Craig,Hubbard, John L.
, p. 1733 - 1734 (1990)
trans-Dichlorobis(triphenylphosphine)rhodium(II), a square planar rhodium(II) monomer, has been isolated and characterized spectroscopically and crystallographically.
Dimeric rhodium-ethylene NHC complexes as reactive intermediates for the preparation of tetra-heteroleptic NHC complexes
Zenkina, Olena V.,Keske, Eric C.,Wang, Ruiyao,Crudden, Cathleen M.
, p. 6423 - 6432 (2011)
Dimeric rhodium complexes with various N-heterocyclic carbene (NHC) ligands have been synthesized and fully characterized. X-ray analysis unambiguously confirms the bimetallic nature of these complexes, and in all cases one molecule of ethylene is coordinated to each metal center in an η2- fashion. The Rh atoms are also coordinated to one NHC ligand and are interconnected by two μ-chlorine bridges. The dimeric nature of the complexes is most likely stabilized due to the significant steric bulk around the metal centers provided by the carbene ligands. Consistent with this, modulating the steric properties and backbone saturation of the ligands was shown to have a significant effect on the stability and geometry of the complexes. Treatment of the carbene dimers with ligands such as PPh3 results in cleavage of the dimers and a unique synthesis of tetra-heteroleptic complexes of the general formula [ClRh(NHC)(PR3)(CH2=CH2)]. The stabilities of these compounds have been assessed, and although decomposition to Wilkinson's complex is observed upon treatment with an excess of phosphine for prolonged times, the presence of the ethylene ligand provides greatly increased stability compared with the bis-phosphine analogues [ClRh(NHC)(PPh 3)2].
Rhodium-catalyzed reductive carbonylation of aryl iodides to arylaldehydes with syngas
Chen, Suqing,Liu, Zhenghui,Mu, Tiancheng,Wang, Peng,Yan, Zhenzhong,Yu, Dongkun,Zhao, Xinhui
, p. 645 - 656 (2020/05/14)
The reductive carbonylation of aryl iodides to aryl aldehydes possesses broad application prospects. We present an efficient and facile Rh-based catalytic system composed of the commercially available Rh salt RhCl3·3H2O, PPh3 as phosphine ligand, and Et3N as the base, for the synthesis of arylaldehydes via the reductive carbonylation of aryl iodides with CO and H2 under relatively mild conditions with a broad substrate range affording the products in good to excellent yields. Systematic investigations were carried out to study the experimental parameters. We explored the optimal ratio of Rh salt and PPh3 ligand, substrate scope, carbonyl source and hydrogen source, and the reaction mechanism. Particularly, a scaled-up experiment indicated that the catalytic method could find valuable applications in industrial productions. The low gas pressure, cheap ligand and low metal dosage could significantly improve the practicability in both chemical researches and industrial applications.
Mechanochemical dehydrocoupling of dimethylamine borane and hydrogenation reactions using Wilkinson's catalyst
Schumacher, Christian,Crawford, Deborah E.,Ragu?, Branimir,Glaum, Robert,James, Stuart L.,Bolm, Carsten,Hernández, José G.
supporting information, p. 8355 - 8358 (2018/08/04)
Mechanochemistry enabled the selective synthesis of the recherché orange polymorph of Wilkinson's catalyst [RhCl(PPh3)3]. The mechanochemically prepared Rh-complex catalysed the solvent-free dehydrogenation of Me2NH·BH3 in a ball mill. The in situ-generated hydrogen (H2) could be utilised for Rh-catalysed hydrogenation reactions by ball milling.
Synthesis method of rhodium(triphenylphosphine)carbonylacetylacetonate
-
Paragraph 0017, (2016/12/01)
The invention discloses a synthesis method of rhodium(triphenylphosphine)carbonylacetylacetonate.The method includes the steps of conducting backflow reaction on rhodium chloride trihydrate and a triphenylphosphine solution to prepare triphenylphosphine rhodium chloride, mixing prepared triphenylphosphine rhodium chloride with N,N-dimethylformamide under the protection of nitrogen, then adding acetylacetone, conducting backflow heating reaction, cooling to the room temperature, concentrating a solution, adding icy water, standing, separating out crystals, conducting filtering, washing filter cakes with water, and conducting vacuum drying to obtain rhodium(triphenylphosphine)carbonylacetylacetonate.By means of the new synthesis method, intermediates are replaced with triphenylphosphine rhodium chloride capable of being stably stored in air, the step-by-step yield and once through yield of the product are increased, and benzene and alkane type toxic solvent are not used; by means of environment-friendly type solvent ethyl alcohol, production cost is reduced, and remarkable economic and environment advantages are achieved.
14694-95-2 Process route
-
![[(triphenylphosphine)3Rh(CH<sub>3</sub>)]](/upload/2023/1/a32f40ea-7a71-431a-85cc-31e173d36172.png)
- 15320-81-7
[(triphenylphosphine)3Rh(CH3)]

-

- 14694-95-2
Wilkinson's catalyst

-

- 15318-33-9,16353-77-8,13938-94-8
chlorocarbonylbis(triphenylphosphine)rhodium(I)

-

- 34731-03-8
{rhodium acetate(PPh3)3}
| Conditions | Yield |
|---|---|
|
With CO2; CH2Cl2; In benzene; High Pressure; a soln. of ((C6H3)3P)3RhCH3 in C6H6 was pressurized with 70 psi of CO2 for 17 h; filtration; evapn. to dryness; dissolving in toluene; filtration; addn. of CH3OH; cooling to -78°C; dissolving of the crystals in CH2Cl2/CD2Cl2;; removal of solvent; dissolving in toluene; filtration; concg. of the filtrate and addn. of CH3OH; cooling at -11°C for 4 weeks; all manipulations were carried out under Ar;;
|
0% |
-

-
rhodium(III) chloride

-

- 603-35-0
triphenylphosphine

-

- 14694-95-2
Wilkinson's catalyst
| Conditions | Yield |
|---|---|
|
In ethanol; at 70 - 75 ℃; for 10h; Large scale;
|
38% |
|
With water; In ethanol;
|
|
|
In water; at 145 ℃; for 0.25h;
|
14694-95-2 Upstream products
-
12081-16-2

dichlorotetraethylene dirhodium (I),
-
603-35-0

triphenylphosphine
-
93860-48-1

(triphenylphosphine)3RhH2Cl
-
15318-33-9

chlorocarbonylbis(triphenylphosphine)rhodium(I)
14694-95-2 Downstream products
-
106-42-3

para-xylene
-
13938-94-8

bis(triphenylphosphine)carbonylrhodium(I) chloride
-
108-88-3

toluene
-
530-26-7

D-Mannose
Relevant Products
-
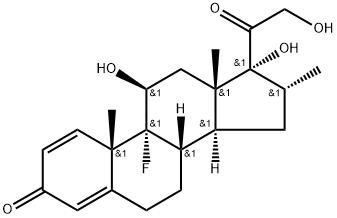
Dexamethasone
CAS:50-02-2
-
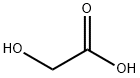
Glycolic acid
CAS:79-14-1