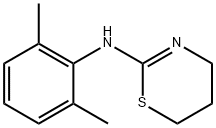
7361-61-7
- Product Name:4H-1,3-Thiazin-2-amine,N-(2,6-dimethylphenyl)-5,6-dihydro-
- Molecular Formula:C12H16N2S
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 7361-61-7
Molecular Formula: C12H16N2S
7361-61-7 Properties
- Molecular Formula:C12H16N2S
- Molecular Weight:220.338
- Melting Point:140 °C
- Refractive Index:1.5700 (estimate)
- Boiling Point:334.2 °C at 760 mmHg
- PKA:7.67±0.20(Predicted)
- Flash Point:155.9 °C
- PSA:49.69000
- Density:1.15 g/cm3
- LogP:2.71680
7361-61-7 Usage
Description
Xylazine is an agonist of α2-adrenergic receptors (Ki = 194 nM). It is an analog of clonidine, an α2-adrenergic receptor agonist used to reduce blood pressure. Xylazine is used for sedation, anesthesia, and analgesia in non-human mammals. This product is also available as an analytical reference standard .
Originator
Xylazine,Bayer
Uses
Antinociceptive;Alpha-2 adrenergic agonist
Uses
Xylazine is an α2 class of adrenergic receptor agonist. Xylazine is a clonidine analoque that acts on presynaptic and postsynaptic receptors as an a 2-adrenergic agonist.
Manufacturing Process
2,6-Dimethylphenyl isothiocyanate, 31.0 g (0.2 mole), prepared from 2,6- dimethylaniline with thiophosgene, were added dropwise during 15 min to a well-stirred suspension of 15.0 g (0.2 mole) of 3-aminopropanol-1 in 100 ml of ether. The ether started to boil. Stirring under reflux was continued for 30 min, and the ether was then distilled off. The residue was treated with 100 ml of concentrated hydrochloric acid and boiled under reflux for 30 min. After cooling, it was diluted with water, filtered free from impurities, and the base was precipitated by the addition of concentrated sodium hydroxide solution. When recrystallized from benzene-ligroin, the resulting compound 2-(2,6- dimethyl-phenylamino)-4H-5,6-dihydro-1,3-thiazine, melting point 140-142°C (yield 90% of the theoretical).
Therapeutic Function
Analgesic, Anesthetic
General Description
Xylazine is soluble in methanol (50 mg/ml), yielding a clear, colorless solution. It is also soluble in dilute HCl acid and in chloroform. Xylazine is practically insoluble in water and in alkali solutions.
Biochem/physiol Actions
Xylazine when used along with ketamine is considered to be a potent and safe anaesthetic in experimental animal. It is known to elevate the hepatic release of glucose, which aggravates to hyperglycemia.
Mechanism of action
Xylazine is marketed as its hydrochloride salt as Rompun (100 mg/mL) and Anased (20 mg/mL) injectable solutions for intravenous administration to horses and dogs, respectively. The actions of xylazine may be reversed by the administration of yohimbine, an indolalkylamine alkaloid, that blocks those α2- adrenoreceptors that are stimulated by xylazine.
Safety Profile
Poison by ingestion, subcutaneous, and intravenous routes. Human systemic effects: change in motor activity, fall in blood pressure, miosis, pleural thickening, pulse rate decrease, somnolence. When heated to decomposition it emits very toxic fumes of NOx and SOx.
InChI:InChI=1/C12H16N2S/c1-9-5-3-6-10(2)11(9)14-12-13-7-4-8-15-12/h3,5-6H,4,7-8H2,1-2H3,(H,13,14)
7361-61-7 Relevant articles
Chemical synthesis method of 2,6-dimethylaniline thiazine
-
Paragraph 0027-0056, (2019/01/16)
The invention discloses a chemical synthesis method of 2,6-dimethylaniline thiazine. The method comprises the following steps: dissolving dried acetyl (2,6-dimethyl) aniline into an anhydrous solution; carrying out quantitative reaction on a mixed solution and carbon disulfide under the action of metal hydride to generate isothiocyanic acid-2,6-dimethyl phenyl ester; carrying out aminolysis on theisothiocyanic acid-2,6-dimethyl phenyl ester and 3-aminopropanol to obtain 1-(2,6-dimethyl)-phenyl-3-propanolyl thiourea after drying and purifying; and catalyzing and cyclizing with concentrated hydrochloric acid to obtain a target product 2,6-dimethylaniline thiazine. The preparation method disclosed by the invention has the advantages of easily-obtained raw materials, rigorous requirements onoperating conditions, simplicity in actual production and suitability for industrial mass production.
Process of preparing an isothiocyanate intermediate used in the preparation of xylazine
-
, (2008/06/13)
A process for preparing 2,6-dimethylphenylisothiocyanate comprises the steps of dissolving 2,6-dimethylaniline in carbon disulfide and ammonium hydroxide to form a dithiocarbamate salt. The salt is reacted with ethyl chloroformate to form 2,6-dimethylphenylisothiocyanate, which may thereafter be reacted with 3-amino-1-propanol and subsequently cyclized, by treating with hydrochloric acid, to form xylazine.
Process for the production of xylazine
-
, (2008/06/13)
In the preparation of 2,6-dimethylphenylisothiocyanate by reacting N-(2,6-dimethylphenyl)acetamide with sodium hydride in an organic solvent to form the corresponding anion of said amide, then reacting carbon disulfide with said anion to form said 2,6-dimethylphenylisothiocyanate, unexpectedly high yields are obtained by using as the organic solvent tetrahydrofuran or a mixture of N,N-dimethylacetamide and toluene. 2,6-Dimethylphenylisothiocyanate is an intermediate in the preparation of xylazine useful, for instance, as a sedative, an analgesic and muscle relaxant.
7361-61-7 Process route
-

-
1-(2,6-dimethylphenyl)-3-(3-propanol)thiourea

-

- 7361-61-7
xylazine
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; at 80 - 95 ℃; Industrial scale;
|
95% |
-

- 87-62-7
2,6-dimethylaniline

-

- 7361-61-7
xylazine
| Conditions | Yield |
|---|---|
|
With acetic anhydride; In tetrahydrofuran; ethanol;
|
113.8 g (84%) |
7361-61-7 Upstream products
-
87-62-7

2,6-dimethylaniline
-
19241-16-8

2,6-dimethylphenylisothiocyanate
-
156-87-6

propan-1-ol-3-amine
-
2198-53-0

N-(2,6-dimethylphenyl)acetamide
7361-61-7 Downstream products
-
40523-99-7

(5,6-dihydro-4H-[1,3]thiazin-2-yl)-(2,6-dimethyl-phenyl)-carbamic acid ethyl ester
-
67057-44-7

2-((Z)-2,6-dimethyl-phenylimino)-[1,3]thiazinane-3-carboxylic acid ethyl ester
-
29280-56-6

N-(5,6-dihydro-4H-[1,3]thiazin-2-yl)-N-(2,6-dimethyl-phenyl)-4-methoxy-benzamide
-
29280-58-8

N-(5,6-dihydro-4H-[1,3]thiazin-2-yl)-N-(2,6-dimethyl-phenyl)-2-methyl-benzamide
Relevant Products
-
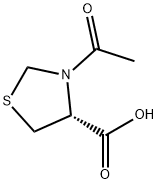
N-Acetyl-L-thioprolineCAS NO.: 54323-50-1
CAS:54323-50-1
-
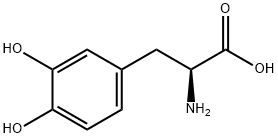
Levodopa
CAS:59-92-7
-
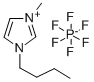
1H-Imidazolium, 3-butyl-1-methyl-, hexafluorophosphate(1-) (1:1)
CAS:174501-64-5