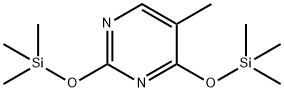
2943-75-1
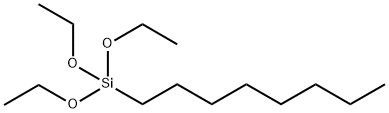
- Product Name:Triethoxyoctylsilane
- Molecular Formula:C14H32O3Si
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 2943-75-1
Molecular Formula: C14H32O3Si
Appearance: colorless transparent liquid
2943-75-1 Properties
- Molecular Formula:C14H32O3Si
- Molecular Weight:276.492
- Appearance/Colour:colorless transparent liquid
- Vapor Pressure:0.137mmHg at 25°C
- Melting Point:-40oC
- Refractive Index:n20/D 1.417(lit.)
- Boiling Point:224.4 °C at 760 mmHg
- Flash Point:101.3 °C
- PSA:27.69000
- Density:0.88 g/cm3
- LogP:4.39530
2943-75-1 Usage
Description
Triethoxyoctylsilane is a monomeric medium-chain alkylfunctional silane. It is a clear colourless liquid and soluble in common non-polar organic solvent. Triethoxyoctylsilane can be used as a surface modifier to generate hydrophobicity (e.g. on concrete, glass, inorganic pigments, or mineral fillers). When diluted with an appropriate solvent, it can be used in the formulation of water repellent products. Upon proper application, the formulated product will penetrate and provide water repellency by chemically reacting with the cementitious substrate. Treated substrates are hydrophobic and retain their original appearance.
Chemical Properties
Colorless transparent liquid
Uses
Triethoxy(octyl)silane is a hydrophobization agent used to limit coalescence in solid-stabilized emulsions.
General Description
Triethoxy(octyl)silane (TEOS) is an alkoxide organosilane with four ethyl ester sidechains. It is used as a self-assembled monolayer and provides a hydrophobic coating with low surface energy. The water contact angle is in the range of 150-170°.
InChI:InChI=1/C14H32O3Si/c1-5-9-10-11-12-13-14-18(15-6-2,16-7-3)17-8-4/h5-14H2,1-4H3
2943-75-1 Relevant articles
-
Cornish,A.J. et al.
, p. 153 - 163 (1979)
-
THE PROCESS FOR THE PREPARATION AND USE OF HAIR TREATMENT COMPOSITIONS CONTAINING ORGANIC C1-C6 ALKOXY SILANES
-
, (2022/01/12)
The subject of the present application is a method for the preparation and use of an agent for the treatment of keratinous material, in particular human hair, comprising the following steps: (1) Mixing one or more organic C1-C6 alkoxy silanes with water,(2) optionally, partial, or complete removal from the reaction mixture of the C1-C6 alcohols liberated by the reaction in step (1),(3) if necessary, addition of one or more cosmetic ingredients,(4) Filling of the preparation into a packaging unit,(5) Storage of the preparation in the packaging unit for a period of at least about 5 days; and(6) Application of the preparation on the keratinous material.
TWO-COMPONENT SYSTEM FOR SMOOTHING AND CARE OF HAIR
-
, (2022/01/23)
-
Efficient magnetically separable heterogeneous platinum catalyst bearing imidazolyl schiff base ligands for hydrosilylation
Huo, Yingpeng,Hu, Jiwen,Tu, Yuanyuan,Huang, Zhenzhu,Lin, Shudong,Luo, Xiaojiong,Feng, Chao
, (2021/02/06)
Reported herein is a magnetically separable heterogeneous nano catalyst Fe3O4@SiO2-biIMI- PtCl2, which is prepared by firstly applying a SiO2 coating onto readily synthesized magnetite nanoparticles via the hydrolysis condensation of tetraethyl orthosilicate (TEOS) under basic conditions, then modifying it using aminopropyl triethoxysilane and bis(imidazole) aldehyde, and finally incorporating a PtCl2 complex via coordination chemistry. The chemical structure and morphology of the nanocatalyst as well as the valence state and content of platinum within this catalyst were carefully characterized. This catalyst can mediate the hydrosilylation between 1-octene and hydrosilane, with the conversion of 1-octene reaching up to 99%, and it shows good regioselectivity as only β-adducts are identified. In addition, this catalyst can be reused for at least 5 cycles. The hydrosilylation reaction between different olefins and hydrosilanes can also be efficiently mediated by Fe3O4@SiO2-biIMI-PtCl2.
Platinum-Pyridine Schiff base complexes immobilized onto silica gel as efficient and low cost catalyst for hydrosilylation
Huo, Yingpeng,Hu, Jiwen,Liu, Feng,Wu, Jiapei,Zhang, Yikun,Zhang, Yalan,Wang, Qianyi
, p. 812 - 818 (2021/07/25)
A heterogeneous platinum catalyst with tridentate pyridine Schiff base ligands supported on silica gel is reported. The catalyst was fully characterized via FTIR, solid-state 13C NMR spectroscopy, X-ray photoelectron spectroscopy (XPS), N2 adsorption/desorption analysis, X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). The catalyst showed potential application in mediating hydrosilylation reactions between olefins and hydrosilanes, and it can be reused for at least five cycles.
2943-75-1 Process route
-

- 111-66-0,25068-25-1
oct-1-ene

-

- 998-30-1
Triethoxysilane

-

- 13389-42-9
trans-2-Octene

-

- 2943-75-1
triethoxy(octyl)silane
| Conditions | Yield |
|---|---|
|
With dodecacarbonyl-triangulo-triruthenium; In 1,4-dioxane; benzene; at 70 ℃; Product distribution; Mechanism; Thermodynamic data; E(a); var. temperatures and Ru3(CO)12 concentrations; also hydrosylilation of acetophenone and 1-octyne;
|
-

- 111-66-0,25068-25-1
oct-1-ene

-

- 998-30-1
Triethoxysilane

-

- 78-10-4
tetraethoxy orthosilicate

-

- 2943-75-1
triethoxy(octyl)silane
| Conditions | Yield |
|---|---|
|
oct-1-ene; Triethoxysilane; With bis(acetylacetonate)nickel(II); In tetrahydrofuran; at 20 ℃; for 0.0166667h; Inert atmosphere;
With sodium triethylborohydride; In tetrahydrofuran; at 50 ℃; for 10h; Inert atmosphere;
|
85% 5% |
2943-75-1 Upstream products
-
78-10-4

tetraethoxy orthosilicate
-
38841-98-4

n-octylmagnesium chloride
-
64-17-5

ethanol
-
5283-66-9

octyltrichlorosilane
2943-75-1 Downstream products
-
67007-46-9

n-octylsilatrane
-
111-87-5

octanol
-
871-92-1

trihydrogeno(octyl)silane
-
357933-40-5

ethyl (2-benzyloxycarbonyl-ethyl)phosphinate
Relevant Products
-
BIS(O-TRIMETHYLSILYL)THYMINE, 98%CAS NO.: 7288-28-0
CAS:7288-28-0
-
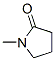
1-Methyl-2-pyrrolidinone
CAS:872-50-4
-
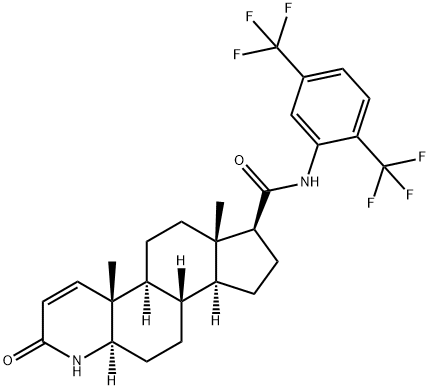
Dutasteride
CAS:164656-23-9