50-28-2
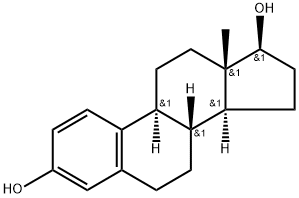
- Product Name:Estra-1,3,5(10)-triene-3,17-diol(17b)-
- Molecular Formula:C18H24O2
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 50-28-2
Molecular Formula: C18H24O2
Appearance: white crystalline powder
50-28-2 Properties
- Molecular Formula:C18H24O2
- Molecular Weight:272.387
- Appearance/Colour:white crystalline powder
- Vapor Pressure:9.82E-09mmHg at 25°C
- Melting Point:178-179 °C(lit.)
- Refractive Index:80.4 ° (C=1, Dioxane)
- Boiling Point:445.917 °C at 760 mmHg
- PKA:pKa 10.71±0.02(H2O(0.1% p-dioxane) t=25±0.1 I=0.03(KCl))(Approximate)
- Flash Point:209.634 °C
- PSA:40.46000
- Density:1.17 g/cm3
- LogP:3.60920
50-28-2 Usage
description
β-Estradiol is an endogenous estrogenic hormone receptor (ER) agonist (Ki values are 0.12 and 0.13 nM for ERα and ERβ respectively). Also high affinity ligand at membrane estrogen GPR30 receptors. β-Estradiol is an activator of PI 3-kinase.Estradiol (17β-estradiol, β-Estradiol, E2, 17β-Oestradiol) is a human sex hormone and steroid, and the primary female sex hormone. Estradiol upregulates IL-6 expression through the estrogen receptor β (ERβ) pathway.
Uses
17β-Estradiol is the major estrogen secreted by the premenopausal ovary.This compound is a contaminant of emerging concern (CECs). Drinking water contaminant candidate list 3 (CCL 3) compound as per United States Environmental Protection Agency (EPA), environmental, and food contaminants.β-Estradiol is used to study cell differentiation and transformations (tumorigenicity).
Indications and Usage
Estradiol is a white or milky white ordorless crystalline powder. It is soluble in dioxane and acetone, slightly soluble in ethanol, and insoluble in water. Estradiol is the intermediate between estradiol valerate and estradiol benzoate, and it is a type of estrogen drug. It can be used to treat uterine functional bleeding, primary amenorrhea, menopausal syndrome, and prostate cancer. Estradiol can promote and adjust the normal growth of female sex organs and secondary sex characteristics, promote mammary duct maturation and growth, and aid in posseting. Estradiol can also be used in biochemical research.
Adverse reactions
In high dosages, estradiol can inhibit the release of anterior pituitary prolactin, thus decreasing breast milk secretion. However, nausea, vomiting and endometrial hyperplasia-induced bleeding may occur. Patients with liver or kidney failure should use with caution.
Contradictions
Do not use on breasts, vaginal area and vaginal mucosa.
Chemical Properties
White or almost white, crystalline powder or colourless crystals.
Chemical Properties
Estradiol, 17-β-is an odorless white to yellow crystalline substance.
Uses
17β-Estradiol is the major estrogen secreted by the premenopausal ovary.This compound is a contaminant of emerging concern (CECs). Drinking water contaminant candidate list 3 (CCL 3) compound as per United States Environmental Protection Agency (EPA), environmental, and food contaminants.
Uses
Estradiol is the major estrogen secreted by the premenopausal ovary.
Uses
Estradiol USP (Estrace) is used to treat Breast cancer; prostatic carcinoma.
Application
β-Estradiol has been used:for the in vitro maturation of bovine cumulus-oocyte complexes (COCs)as a supplement in in vitro maturation medium (IVM), which is used as a control mediumin estrogen-induction assay
Definition
ChEBI: The 17beta-isomer of estradiol.
Acquired resistance
Estradiol is the most potent endogenous estrogen, exhibiting high affinity for the ER and high potency when administered parenterally. When administered orally, estradiol is promptly conjugated in the intestine and oxidatively metabolized by the liver, resulting in its low oral bioavailability and therapeutic effectiveness.
General Description
Estradiol, estra-1,3,5(10)-triene-3,17β-diol, is the most activeof the natural steroid estrogens. Although its 17β-OHgroup is vulnerable to bacterial and enzymatic oxidation toestrone, it can be temporarily protected as anester at C3 or C17, or permanently protected by adding a17α-alkyl group (e.g., 17α-ethinyl estradiol, the most commonlyused estrogen in oral contraceptives). The increasedoil solubility of the 17β-esters (relative to estradiol) permitsthe esters to remain in oil at the IM injection site for extendedperiods. These derivatives illustrate the principles of steroidmodification. Transdermal estradiolproducts avoid first-pass metabolism, allowing estradiol tobe as effective as oral estrogens for treating menopausalsymptoms. A new transdermal spray, Evamist, was approvedin 2007. Estradiol itself is typically not very effective orallybecause of rapid metabolism, but an oral formulation of micronizedestradiol that allows more rapid absorption of thedrug is available (Estrace). In addition to the oral and transdermalproducts, estradiol is also available in gel, cream, andvaginal ring formulations. The commercially available estradiolesters are the following:Estradiol 3-acetate, USP (oral; vaginal ring)Estradiol 17-valerate, USP (IM injection)Estradiol 17-cypionate, USP (IM injection).
Hazard
A carcinogen (OSHA).
Biological Activity
Endogenous estrogen receptor (ER) agonist (K i values are 0.12 and 0.13 nM for ER α and ER β respectively). Also high affinity ligand at membrane estrogen GPR30 receptors.
Contact allergens
Natural estradiol, used in transdermal systems for hormonal substitution, can induce allergic contact dermatitis, with the risk of systemic contact dermatitis after oral reintroduction.
Biochem/physiol Actions
The major estrogen secreted by the premenopausal ovary. Estrogens direct the development of the female phenotype in embryogenesis and during puberty by regulating gene transcription and, thus, protein synthesis. It also induces the production of gonadotropins which, in turn, induce ovulation. Exposure to estradiol increases breast cancer incidence and proliferation.
Mechanism of action
The most potent naturally occurring estrogen in mammals. It is synthesized primarily in the ovary, and also in the testis, adrenal gland and placenta, and to a limited extent by peripheral tissues (e.g., liver, fat, and skeletal muscle) from androstenedione and testosterone. It is responsible for the development of secondary sex characteristics in the female at puberty (i.e., growth and development of the vagina, uterus and fallopian tubes, enlargement of the breasts, and growth and maturation of long bones).
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. A promoter. Human reproductive effects by ingestion: ferthty effects. Experimental reproductive effects. Human mutation data reported. A steroid hormone much used in medicine. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
Estradiol, estra-1,3,5(10)-trien-3,17β-diol (28.1.17), is most easily made by reducing the keto-group of estrone by various reducing agents, in particular potassium borohydride.
Potential Exposure
The working environment may be contaminated during sex hormone manufacture, especially during the extraction and purification of natural steroid hormones; grinding of raw materials; handling of powdered products and recrystallization. Airborne particles of sex hormones may be absorbed through the skin, ingested or inhaled. Enteric absorption results in quick inactivation of sex hormones in the liver. The rate of inactivation is decreased for the oral, alkylated steroid hormones (methyl testosterone, anabolic steroids, etc.). Sex hormones may accumulate and reach relatively high levels even if their absorption is intermittent. Consequently, repeated absorption of small amounts may be detrimental to health. Intoxication by sex hormones may occur in almost all the exposed workers if preventive measures are not taken. The effect in the industrial sector is more successful than the agricultural one (chemical caponizing of cockerels by stilbestrol implants and incorporation of estrogens in feed for body weight gain promotion in beef cattle), where measures taken are summary and the number of cases of intoxication is consequently bigger
Shipping
UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
17-Estradiol (previously known as -estradiol) is purified by chromatography on SiO2 (toluene/EtOAc 4:1) and recrystallised from CHCl3/hexane or 80% EtOH. It is stable in air, is insoluble in H2O, and is precipitated by digitonin. The UV has max at 225 and 280 nm. The diacetate [3434-88-6] has m 97-98o and forms leaflets from aqueous EtOH. The 3-benzoate crystallises from aqueous MeOH withm 193o and [] D 25 +58o to 63o (c 1, dioxane). [Meischer & Scholz Helv Chim Acta 20 263, 1237 1937, Biochem J 32 1273 1938, Oppolzer & Roberts Helv Chim Acta 63 1703 1980, Inhoffen & Zühlsdorff Chem Ber 7 4 1914 1941, Beilstein 6 IV 6611.]
InChI:InChI=1/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m0/s1
50-28-2 Relevant articles
ANDROGEN METABOLISM IN MALE AND FEMALE BREAST TISSUE
Perel, E.,Davis, S.,Killinger, D. W.
, p. 345 - 352 (1981)
Incubation studies have been carried out using normal breast tissue and breast tissue from patients with gynecomastia, mammary dysplasia and breast carcinoma to determine the pattern of androstenedione metabolism.All tissues formed estrone (E1) and testosterone (T) in all incubations.Estradiol (E2) was isolated in incubations of tissue from 1 of 6 patients with mammary dysplasia, 5 of 6 patients with gynecomastia and in all incubations with normal and carcinoma tissue.Estrone formation was lowest in mammary dysplasia and gynecomastia, and higher in apparently normal breast tissue.The greatest E1 formation was found in incubations with breast carcinoma tissue, although there was considerable variation within this tissue group.Estradiol formation was low in all tissues, with the highest conversion rates in carcinoma tissue.Testosterone formation in carcinoma tissue was greater than in mammary dysplasia or gynecomastia, but similar to apparently normal tissue.These results indicate that breast tissue from different pathological states varies in its capacity to aromatize androstenedione (A) to estrogenic products and to convert it to other androgens.They have also shown that the pattern of metabolism is distinctive for the nature of the pathological abnormality.
-
MacCorquodale,Thayer,Doisy
, p. 435,438 (1936)
-
Mechanism of action of bolandiol (19-nortestosterone-3β,17β-diol), a unique anabolic steroid with androgenic, estrogenic, and progestational activities
Attardi, Barbara J.,Page, Stephanie T.,Hild, Sheri A.,Coss, Christopher C.,Matsumoto, Alvin M.
, p. 151 - 161 (2010)
Bolandiol is a synthetic anabolic steroid that increases lean body mass and bone mineral density without significant stimulation of sex accessory glands in castrate adult male rats. Since bolandiol suppresses gonadotropins and endogenous testosterone (T) production, we investigated its mechanism of action. We compared the potency of bolandiol in vitro and in vivo with T, 5α-dihydrotestosterone (DHT), 19-nortestosterone (19-NT) and estradiol (E2). Bolandiol bound with lower affinity to the recombinant rat androgen receptor (AR) than the other androgens and had low, but measurable, affinity for recombinant human progestin receptors (PR-A, PR-B), and estrogen receptors (ERα and β-1). Functional agonist activity was assessed in transcription assays mediated by AR, PR, or ER. Bolandiol was stimulatory in all these assays, but only 4-9% as potent as T, DHT, and 19-NT via AR, 1% as potent as progesterone via PR, and 3% and 1% as potent as E2 acting through ERα or ERβ, respectively. In immature castrate rats, bolandiol was equipotent to T in stimulating growth of the levator ani muscle but less potent than T in stimulating growth of the sex accessory glands. Bolandiol also stimulated uterine weight increases in immature female rats, which were partly blocked by ICI 182,780, but it was not aromatized in vitro by recombinant human aromatase. In contrast to T, stimulation of sex accessory gland weights by bolandiol was not inhibited by concomitant treatment with the dual 5α-reductase inhibitor dutasteride. As bolandiol exhibits tissue selectivity in vivo, it may act via AR, PR, and/or ER, utilize alternative signaling pathway(s) or transcriptional coregulators, and/or be metabolized to a more potent selective steroid.
Substrate specificity of the placental microsomal aromatase
Gibb,Lavoie
, p. 507 - 519 (1980)
Using an accurate and sensitive assay for the human placental aromatase we apparent found apparant Km values for androstenedione (4-androstene-3, 17-dione) and testosterone to be 14 ± 4.0 nM and 41 ± 12 nM respectively. These values were significantly different (p 0.001). Analyses at substrate concentrations 5-10 fold above and below the Km values did not indicate any anomalous kinetic behavior. Mixed substrate experiments were consistent with a single enzyme metabolizing both steroids; each competitively inhibited the aromatization of the other, and the 'Ki' values were the same as their apparent Km values. Sodium chloride (1.2M) significantly increased the rate of testosterone aromatization by decreasing its Km value and had no significant effect on the aromatization of androstenedione. However, in the presence of this salt testosterone still inhibited the aromatization of androstenedione competitively with a 'Ki' equal to its apparent Km. Our data is therefore consistent with the proposal that human placental microsomes contain a single 'high affinity' site for the aromatization of androstenedione and testosterone.
-
Whitman,Wintersteiner,Schwenk
, p. 789,794 (1937)
-
Aldo-keto reductase 1C3 expression in MCF-7 cells reveals roles in steroid hormone and prostaglandin metabolism that may explain its over-expression in breast cancer
Byrns, Michael C.,Duan, Ling,Lee, Seon Hwa,Blair, Ian A.,Penning, Trevor M.
, p. 177 - 187 (2010)
Aldo-keto reductase (AKR) 1C3 (type 5 17β-hydroxysteroid dehydrogenase and prostaglandin F synthase), may stimulate proliferation via steroid hormone and prostaglandin (PG) metabolism in the breast. Purified recombinant AKR1C3 reduces PGD2 to 9α,11β-PGF2, Δ4-androstenedione to testosterone, progesterone to 20α-hydroxyprogesterone, and to a lesser extent, estrone to 17β-estradiol. We established MCF-7 cells that stably express AKR1C3 (MCF-7-AKR1C3 cells) to model its over-expression in breast cancer. AKR1C3 expression increased steroid conversion by MCF-7 cells, leading to a pro-estrogenic state. Unexpectedly, estrone was reduced fastest by MCF-7-AKR1C3 cells when compared to other substrates at 0.1 μM. MCF-7-AKR1C3 cells proliferated three times faster than parental cells in response to estrone and 17β-estradiol. AKR1C3 therefore represents a potential target for attenuating estrogen receptor α induced proliferation. MCF-7-AKR1C3 cells also reduced PGD2, limiting its dehydration to form PGJ2 products. The AKR1C3 product was confirmed as 9α,11β-PGF2 and quantified with a stereospecific stable isotope dilution liquid chromatography-mass spectrometry method. This method will allow the examination of the role of AKR1C3 in endogenous prostaglandin formation in response to inflammatory stimuli. Expression of AKR1C3 reduced the anti-proliferative effects of PGD2 on MCF-7 cells, suggesting that AKR1C3 limits peroxisome proliferator activated receptor γ (PPARγ) signaling by reducing formation of 15-deoxy-Δ12,14-PGJ2 (15dPGJ2).
INHIBITION OF ESTROGEN SYNTHESIS IN HUMAN BREAST TUMORS BY TESTOLOLACTONE AND BROMOANDROSTENEDIONE
Budnick, Rose Marie,Dao, Thomas L.
, p. 533 - 542 (1980)
The inhibition of aromatase enzyme in human breast tumors by Δ1-testololactone, testololactone, 6α-bromoandrostenedione, and 6β-bromoandrostenedione was investigated.Estrone and estradiol synthesis from androstenedione was reduced in 3 tumor incubations by the presence of 0.13 mmol Δ1-testololactone and testololactone. 6α- and 6β-bromoandrostenedione (2.0 μM) were also shown to block estrogen synthesis in 2 tumors.Furthermore, Lineweaver-Burk plots revealed that all 4 compounds are competitive inhibitors of androstenedione aromatization.An apparent Km of the aromatase enzyme for androstenedione of 0.08 μM and a Vmax of 23 pmol of estrone synthesized/g tumor/hr were determined for one human breast tumor specimen.These results demonstrate that these aromatase inhibitors may be useful for the treatment of breast cancer.
Complexation of steroid hormones with cyclodextrin derivatives: Substituent effects of the guest molecule on solubility and stability in aqueous solution
Albers,Muller
, p. 756 - 761 (1992)
The inclusion complexation of homologous derivatives of steroid hormones with cyclodextrins and 2-hydroxypropyl-β-cyclodextrin (2-HP-β-CD) was investigated with regard to underlying structure-interaction relationship. The interaction was studied by phase solubility analysis and stabilization effects of complex formation with 2-HP-β-CD. The solubilizing and stabilizing abilities of 2-HP-β-CD were generally more effective for testosterone derivatives than for estradiol esters. Within a homologous series of steroid hormones, the steepest linear solubility isotherms were found for 17-methyl and 3-methyl derivatives. The solubilization of steroid esters by 2-HP-β-CD depended on the structure and length of the ester side chain. The interaction of 2-HP-β-CD with the steroids was hindered by long- chain fatty acid ester groups. With increasing length of the side chain, a decline of the isotherms occurred and the phase solubility behavior changed from linear to exponential. Contrary to expectations, benzoylation of steroids considerably decreased the guest-host interaction. The observed rates of degradation of the steroid esters were significantly reduced by 2- HP-β-CD, depending on the chain length, and correlated well with the order found in phase solubility analysis. The degradation showed no deviations from pseudo-first-order kinetics, and the degradation mechanism was not changed because of complexation. The results suggest that interaction of 2-HP-β-CD with steroid esters involves the ester functions of the prodrugs and is more suitable for unsubstituted guest molecules.
Thermodynamic Meerwein-Ponndorf-Verley reduction in the diastereoselective synthesis of 17α-estradiol
Ahmed, Gulzar,Nickisch, Klaus
, p. 1 - 4 (2016)
The synthesis of 17α-hydroxy steroids generally requires multiple synthetic manipulations. The synthesis of 17α-estradiol is no exception, as this process involves the protection and release of the 3-hydroxy functional group. The diastereoselective reduction of the 17-keto-steroid can be utilized to prepare 17α-hydroxy-steroids. Here, 17α-estradiol was synthesized from commercially available estrone under thermodynamic Meerwein-Ponndorf-Verley (MPV) conditions in a single step, followed by simple chromatographic separation over silica gel. The remaining mixture of unreacted estrone and estradiols was easily recycled through Oppenauer oxidation to estrone, with an overall yield of 68% 17α-estradiol.
177. The Enantioselective Synthesis of (+)-Estradiol from 1,3-Dihydrobenzothiophene-2,2-dioxide by Successive Thermal SO2-Extrusion and Cycloaddition Reactions
Oppolzer, Wolfgang,Roberts, David Anthony
, p. 1703 - 1705 (1980)
The optically pure steroid (+)-15 has been synthesized from the easily accessible (+)-carboxylic acid 11 by a sequence of 7 steps in 50percent overall yield.The key steps are the regioselective deprotonation/alkylation 7+13->14 and the thermal SO2-extrusion/cycloaddition 14->15 (Scheme 3).The compound (+)-15 has been readily converted to the naturally occurring (+)-estradiol (17) in 60percent yield.
Estramustine binding in rat, baboon and human prostate measured by high pressure liquid chromatography
Kirdani,Corrales,Hoisaeter,Karr,Murphy,Sandberg
, p. 471 - 484 (1981)
High pressure liquid chromatography (HPLC) was used to determine 3H-estramustine (estradiol-17β3N-bis-[2-chlorethyl] carbamate), 3H-17β-hydroxy-5α-androstan-3-one (3H-dihydrotestosterone or 3H-DHT), 3H-estradiol-17β (3H-E2) and 3H-3β-hydroxy-5-pregnen-20-one (3H-pregnenolone) binding in 50μl of cytosol utilizing a column which separates proteins in the molecular weight range of 2,000 to 70,000 daltons. The rat prostate contains a protein in considerable concentration and with the highest affinity for estramustine (375,000dpm 3H-estramustine per mg. cytosol protein) among the substances tested. Operationally, we have named this protein 'estramustine binding protein' (EBP), though it is very likely similar to other previously described prostatic proteins (e.g., α-protein, prostatein, prostatic binding protein). The sensitivity of the HPLC method disclosed EBP-like proteins, but in much lesser concentrations, in some of the other tissues tested. The concentration of these proteins in the human and baboon prostates was much lower (average for the baboon cranial lobe 4800dpm/mg cytosol protein, with a somewhat higher value for the caudal lobe) than that in the rat gland. The amount of the EBP-like protein was higher in prostatic cancer than in that of benign prostatic hypertrophy (BPH) (range 9350 - 25,900 vs. 2200 - 18,900 dpm/mg cytosol protein). In the human, the highest value was found in one normal prostate tested (106,000 dpm/mg) cytosol protein).
Purification and characterization of aromatase from human placenta
Hall, Peter F.,Chen, Shiuan,Nakajin, Shizuo,Shinoda, Masato,Shively, John E.
, p. 37 - 50 (1987)
Aromatase from human placenta has been purified to homogeneity (MW 55000).Enzymatic activity can be reconstituted with reductase from pig liver in an aqueous buffer or after incorporation of the enzyme into liposomes.In both cases the enzyme converts androstenedione to estrone and testosterone to estradiol.Aromatase shows a typical CO-spectrum when reduced with dithionite and a type I spectral shift with both substrates.The NH2 terminal amino acid sequence is hydrophobic but shows no homology to that of other cytochromes P-450.Five cysteine peptides have been isolated by HPLC following tryptic digestion of the -carboxymethylated protein.Amino acid sequence of these peptides reveal that histidine is the carboxy-terminal amino acid of the protein and that significant homology exists with corresponding peptides from other cytochromes P-450.Unique oligonucleotides (62 and 30 MER) synthesized on the basis of a 45 amino acid sequence near the center of the molecular have been used to clone the aromatase gene from a cDNA expresssion library from human placenta in λgt11.
An environmentally friendly and cost effective synthesis of estradiol featuring two novel reagents: Si(0)/KF and PMHS/hexamethyldisiloxane/pTSA
Lim, Chongsoo,Evenson, Gerald N.,Perrault, William R.,Pearlman, Bruce A.
, p. 6417 - 6420 (2006)
Si(0)/KF is introduced as a strong, inexpensive, environmentally friendly, and safe reagent for 'dissolving metal'-type reduction. PMHS/hexamethyldisiloxane/pTSA is introduced as an inexpensive substitute for Et3SiH/TFA for 'ionic hydrogenation', where the hexamethyldisiloxane functions as a capping agent to block the oligomeric silicone by-product from cross-linking to a gel, rubber, or plastic. An environmentally friendly and cost effective synthesis of estradiol is described which showcases these new reagents.
A COMPARISON OF THREE METHODS OF HYDROLYSIS FOR ESTROGEN CONJUGATES
Bain, J. D.,Kasman, L. H.,Bercovitz, A. B.,Lasley, B. L.
, p. 603 - 620 (1984)
The efficiencies for estrogen conjugate hydrolysis were compared between enzyme hydrolysis, acid solvolysis and a new method, ammonolysis.Samples included: 1) crystalline 1,3,5(10)-estratriene-3,17β-diol disulfate (estradiol 3,17-disulfate), 2) squirrel monkey urine collected following an intravenous injection of 1,3,5(10)-estratriene-3,17β-diol (estradiol) and 3) a pool of human pregnancy urine.Ammonolysis demonstrated a significant increase over the other techniques in "free" estrogen yields, specifically, from estradiol 3,17-disulfate.
Catalytic properties of pristine and defect-engineered Zr-MOF-808 metal organic frameworks
Mautschke,Drache,Senkovska,Kaskel,Llabrés Xamena
, p. 3610 - 3616 (2018)
Various defect-engineered Zr-trimesate MOF-808 compounds (DE-MOF-808) have been prepared by mixing the tricarboxylate ligands with dicarboxylate ligands; viz. isophthalate, pyridine-3,5-dicarboxylate, 5-hydroxy-isophthalate, or 5-amino-isophthalate. The resulting mixed-ligand compounds, MOF-808-X (X = IP, Pydc, OH or NH2) were all found to be highly crystalline and isostructural to the unmodified MOF-808. Pristine MOF-808 showed better catalytic performance than a UiO-66 reference compound for the Meerwein-Ponndorf-Verley (MPV) reduction of carbonyl compounds. This was attributed to a higher availability of coordinatively unsaturated Zr4+ sites (cus) in MOF-808 upon removal of formate ions. Meanwhile, cus in UiO-66 are only located at defect sites and are thus much less abundant. Further improvement of the catalytic activity of defect-engineered MOF-808-IP and MOF-808-Pydc was observed, which may be related with the occurrence of less crowded Zr4+ sites in DE-MOF-808. The wider pore structure of MOF-808 with respect to UiO-66 compounds translates into a sharp improvement of the activity for the MPV reduction of bulky substrates, as shown for estrone reduction to estradiol. Interestingly, MOF-808 produces a notable diastereoselectivity towards the elusive 17-α-hydroxy estradiol.
6α-Fluorotestosterone: a nonaromatizable androgen inhibitor of aromatase cytochrome P450
Kellis, James T.,Vickery, Larry E.
, p. 242 - 246 (1990)
In an early survey of steroids which might serve as estrogen precursors, Gual et al. reported that 6α-fluorotestosterone is not aromatized by human placental microsomes.Subsequently, 6α-fluorotestosterone has been used to distinguish between androgen- and estrogen-mediated physiologic effects.We have reexamined the interaction of 6α-fluorotestosterone with human placental and rat ovarian microsomes and with reconstituted purified aromatase cytochrome P450.Under conditions in which testosterone was readily aromatized, no aromatization of 6α-fluorotestosterone was observed using either fluorescence detection of dansyl-estrogens separated by high-performance liquid chromatography or estrogen radioimmunoassay methods.The lack of aromatization is not due to failure of 6α-fluorotestosterone to bind P450arom, because 6α-fluorotestosterone acts as a competitive inhibitor of the enzyme, and it exhibits a binding affinity similar to that of testosterone.Moreover, the addition of 6α-fluorotestosterone to human placental microsomes elicits a spectral shift indicative of conversion of the heme from a low to a high spin state as observed for androgen substrates, cosistent with its binding to the substrate site.The mechanism by which substitution of a fluorine at the 6α-position interferes with the aromatization reaction remains to be determined, but the inhibitory action on estrogen formation may potentiate the androgenic properties of 6α-fluorotestosterone in vivo due to a lowering of estrogen levels.
Promiscuity of an unrelated anthrol reductase ofTalaromyces islandicusWF-38-12
Singh, Shailesh Kumar,Rajput, Anshul,De, Arijit,Chakraborti, Tapati,Husain, Syed Masood
, p. 474 - 478 (2021/02/09)
An anthrol reductase ofTalaromyces islandicusWF-38-12 (ARti-2) from an unrelated biosynthetic gene cluster (BGC) has been identified and characterized. It catalyses the NADPH-dependent reduction of anthrols (hydroanthraquinones), estrone and a naphthol with high stereo- and regioselectivity. The role of ARti-2, theCRG89872.1gene of the same BGC and non-enzymatic oxidation in the biosynthesis of (?)-flavoskyrin has been proposed.
One-Step Chemo-, Regio- and Stereoselective Reduction of Ketosteroids to Hydroxysteroids over Zr-Containing MOF-808 Metal-Organic Frameworks
Llabrés i Xamena, F. X.,Mautschke, H.-H.
, p. 10766 - 10775 (2021/06/15)
Zr-containing MOF-808 is a very promising heterogeneous catalyst for the selective reduction of ketosteroids to the corresponding hydroxysteroids through a Meerwein-Ponndorf-Verley (MPV) reaction. Interestingly, the process leads to the diastereoselective synthesis of elusive 17α-hydroxy derivatives in one step, whereas most chemical and biological transformations produce the 17β-OH compounds, or they require several additional steps to convert 17β-OH into 17α-OH by inverting the configuration of the 17 center. Moreover, MOF-808 is found to be stable and reusable; it is also chemoselective (only keto groups are reduced, even in the presence of other reducible groups such as C=C bonds) and regioselective (in 3,17-diketosteroids only the keto group in position 17 is reduced, while the 3-keto group remains almost intact). The kinetic rate constant and thermodynamic parameters of estrone reduction to estradiol have been obtained by a detailed temperature-dependent kinetic analysis. The results evidence a major contribution of the entropic term, thus suggesting that the diastereoselectivity of the process is controlled by the confinement of the reaction inside the MOF cavities, where the Zr4+ active sites are located.
Potent aromatase inhibitors through fungal transformation of anti-cancer drug testolactone: An approach towards treatment of breast cancer
-
Paragraph 0021, (2021/07/30)
Biotransformation of an aromatase inhibitor, testolactone (1), yielded five new metabolites, 7α-hydroxy-3-oxo-13,17-secoandrosta-1,4-dieno-17,13α-lactone (2), 7β-hydroxy-3-oxo-13,17-seco-5β-androsta-1-eno-17,13α-lactone (3), 3α,11β-dihydroxy-13,17-seco-5β-androsta-17,13α-lactone (4), 4β,5β-epoxy-3β-hydroxy-13,17-secoandrosta-1-eno-17,13α-lactone (5), and 4β,5β-epoxy-3α-hydroxy-13,17-secoandrosta-1-eno-17,13α-lactone (6). Aromatase (estrogen synthase) involves in the synthesis of estrogen, and promotes the growth of breast cancerous cells. It is a key target for the discovery of chemotherapeutic agents against ER+ (estrogen-positive) breast-cancers and several other diseases caused by overexpression of aromatase enzyme. Metabolites 3 (IC50=8.60±0.402 nM), and 4 (IC50=9.23±1.31 nM) were identified as potent inhibitors against human aromatase enzyme, in comparison to 1 (IC50=0.716±0.031 μM), and the standard aromatase inhibiting drug, exemestane (IC50=0.232±0.031 μM). Derivatives 2 (IC50=11.68±0.73 μM), 5 (IC50=10.37±0.50 μM) and 6 (IC50=0.82±0.059 μM) have also a good inhibition against aromatase enzyme. Therefore, metabolites 2-6 have the potential to serve as therapeutic agents against diseases caused by aromatase enzyme, including breast cancer.
Solvent- and Wavelength-Dependent Photolysis of Estrone
Adriano, Natalie,Ahearn, Ceilidh,Black, Cory,Cracchiolo, Michael,Ghere, Daniel,Hare, Patrick M.,Nu?ez, Alexandra,Olivan, Lars,Patel, Raj,Saner, Stephanie,Smith, Krista R.,Watkins, Barbie
, (2021/11/08)
The direct photolysis of estrone in solvents ranging from water to cyclohexane is reported. The photodegradation is dominated by lumiestrone, an epimer of estrone resulting from the inversion of the methyl group at carbon 13, regardless of solvent and pho
50-28-2 Process route
-

- 63-05-8
Androstenedione

-

- 50-28-2
estradiol

-

- 53-16-7
Estrone

-

- 58-22-0
testosterone

-

- 521-18-6
Stanolone

-

- 53-42-9
Etiocholanolone

-

- 53-41-8
cis-androsterone
| Conditions | Yield |
|---|---|
|
With carcinoma; gynecomastia; mammary dysplasia; at 37 ℃; for 1.5h; Product distribution; cofactors under 95percent O2: 5percent CO2, <3H>labeled study;
|
-

- 157231-19-1
17β-estradiol 3'-(saccharinylmethyl) ether

-

- 50-00-0,30525-89-4,61233-19-0
formaldehyd

-

- 50-28-2
estradiol

-

- 81-07-2
saccharin
| Conditions | Yield |
|---|---|
|
With phosphate buffer; water; In methanol; at 37 ℃; Rate constant; various pH-values (4.98-7.98); half-life; other media, other temperature;
|
50-28-2 Upstream products
-
91-22-5

quinoline
-
846-48-0

1-dehydrotestosterone
-
99-35-4

1,3,5-trinitrobenzene
-
53-16-7

Estrone
50-28-2 Downstream products
-
1474-52-8

17β-oestradiol diacetate
-
1743-60-8

17-β-estradiol 17-acetate
-
24381-12-2

2-iodo-17β-oestradiol
-
15833-07-5

2-bromo-17β-oestradiol
Relevant Products
-
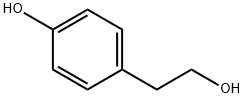
4-Hydroxyphenethyl alcoholCAS NO.: 501-94-0
CAS:501-94-0
-
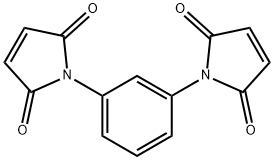
N,N'-1,3-Phenylene bismaleimide
CAS:3006-93-7
-
Estradiol valerate
CAS:979-32-8