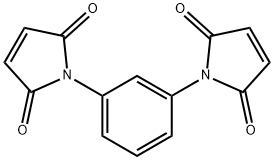
3006-93-7
- Product Name:N,N'-1,3-Phenylene bismaleimide
- Molecular Formula:C14H8N2O4
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 3006-93-7
Molecular Formula: C14H8N2O4
Appearance: yellow crystalline powder
3006-93-7 Properties
- Molecular Formula:C14H8N2O4
- Molecular Weight:268.229
- Appearance/Colour:yellow crystalline powder
- Melting Point:198-201 °C(lit.)
- Refractive Index:1.5910 (estimate)
- Boiling Point:499.3 °C at 760 mmHg
- PKA:-1.67±0.20(Predicted)
- Flash Point:250.7 °C
- PSA:74.76000
- Density:1.567 g/cm3
- LogP:0.67540
3006-93-7 Usage
Chemical Properties
yellow crystalline powder
Uses
rubber vulcanizing agent; rubber crosslinkimg agent
Uses
N,N'-1,3-Phenylene bismaleimide and sulfur coordination to prevent reversion to improve the heat resistance, reduce heat, anti-aging, improve adhesion of the rubber and tire cord and vulcanized rubber modulus.PDM is a non-sulfur vulcanizing agents solve copper wire and copper electrical contacts because of sulfur vulcanizing agent to generate black copper sulfide pollution problems.
Flammability and Explosibility
Notclassified
3006-93-7 Relevant articles
A N, inter-N' - phenylene bismaleimide preparation method
-
Paragraph 0027, (2019/07/04)
The invention discloses a N, inter-N' - phenylene bismaleimide preparation method, which belongs to the technical field of fine chemicals. The raw material is toluene, maleic anhydride and the polymerization inhibitor, heated to 40 - 60 °C dissolve; the metaphenylene diamine dissolved in strong polar solvent then drop added in a reaction vessel, and milling after thermal insulation, adding catalyst heating temperature to reflux, until no more water is stopped when the reaction is separated out; the reaction cooling after crystallization, filtered, washed with water, to obtain pale yellow solid N, between the N' - phenylene bismaleimide. The invention two-step reaction in the same reaction in the container, does not need to separation and purification of the intermediate product, its synthesis process is simple in operation, the working environment is obviously improved. The sold product liquid chromatography the content of 75% -80%, for the production of this invention liquid chromatography content can be up to 90% or more, the purity is higher than current commercial products.
N, inter-N' - phenylene bismaleimide and its preparation method
-
Paragraph 0016; 0017; 0018; 0019; 0020; 0021; 0022-0030, (2017/05/10)
The invention provides a preparation method of a multifunctional rubber vulcanizer N,N'-m-phenylenedimaleimide under the condition of no acetic anhydride. M-phenylenediamine, cis butenyl maleic anhydride, toluene and ethyl acetate are used as raw materials to synthesize the N,N'-m-phenylenedimaleimide. The reaction process is simple and does not need acid anhydride or any other catalyst; and the obtained product has high purity.
Process for the preparation of aromatic maleimides
-
, (2008/06/13)
Aromatic maleimides can be prepared by reacting corresponding aromatic amines with maleic anhydride in the presence of a solvent mixture of halogenated hydrocarbon and a dipolar aprotic solvent, with the addition of acid and a polymerization inhibitor.
Functional monomaleimide and thermosetting composition therefrom
-
, (2008/06/13)
Novel N-(meth)allyloxyphenylmaleimides are prepolymerized with at least one bis-imide and formulated, together with at least one imidazole compound, into thermosetting compositions useful for the production of a wide variety of shaped articles having improved mechanical properties.
3006-93-7 Process route
-

- 108-31-6
maleic anhydride

-

- 16674-78-5
magnesium(II) acetate tetrahydrate

-

- 3006-93-7
N,N'-(1,3-phenylene)dimaleimide
| Conditions | Yield |
|---|---|
|
With acetic anhydride; In water; acetone;
|
84% |
-

- 108-31-6
maleic anhydride

-

- 108-45-2,64554-26-3,76583-20-5
m-phenylenediamine

-

- 3006-93-7
N,N'-(1,3-phenylene)dimaleimide
| Conditions | Yield |
|---|---|
|
In ethyl acetate; toluene; at 60 ℃; for 5h; Time; Temperature;
|
3006-93-7 Upstream products
-
108-31-6

maleic anhydride
-
16674-78-5

magnesium(II) acetate tetrahydrate
-
108-45-2

m-phenylenediamine
-
13161-99-4

S01819
3006-93-7 Downstream products
-
113452-03-2

C36H24N2O6
Relevant Products
-
Benzene,1,1'-(1,1,2,2-tetramethyl-1,2-ethanediyl)bis-
CAS:1889-67-4
-
Estra-1,3,5(10)-triene-3,17-diol(17b)-
CAS:50-28-2