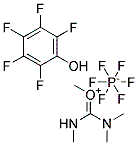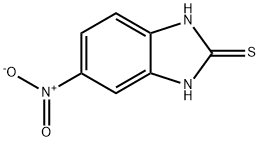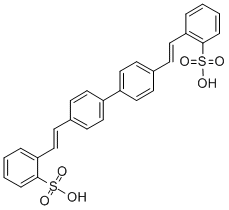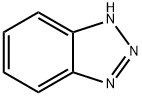
95-14-7
- Product Name:1H-BenzotriazoleCAS NO.: 95-14-7
- Molecular Formula:C6H5N3
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 95-14-7
Molecular Formula: C6H5N3
Appearance: colourless liquid
Delivery Time: In stock
Throughput: 10000|Gram|Day
Purity: 99%
| Chemical Properties | yellow to beige solid |
| Uses | Benzotriazole (BT) is an anticorrosive agent well known for its use in aircraft deicing and antifreeze fluids but also used in dishwasher detergents. in vivo. |
| Definition | ChEBI: The simplest member of the class of benzotriazoles that consists of a benzene nucleus fused to a 1H-1,2,3-triazole ring. |
| General Description | White to light tan crystals or white powder. No odor. |
| Air & Water Reactions | Dust may form an explosive mixture in air. Slightly soluble in water. |
| Reactivity Profile | The triazoles are a group of highly explosive materials that are sensitive to heat, friction, and impact. Sensitivity varies with the type substitution to the triazole ring. Metal chelated and halogen substitution of the triazol ring make for a particularly heat sensitive material. Azido and nitro derivatives have been employed as high explosives. No matter the derivative these materials should be treated as explosives. |
| Hazard | Highly toxic by ingestion. May explode under vacuum distillation. |
| Health Hazard | ACUTE/CHRONIC HAZARDS: When heated to decomposition 1H-Benzotriazole emits toxic fumes. 1H-Benzotriazole can react violently during vacuum distillation. |
| Fire Hazard | Flash point data are not available for 1H-Benzotriazole. 1H-Benzotriazole is probably combustible. |
| Carcinogenicity | Chronic (2-year) feeding studies were conducted. Rats were given 0, 6700, or 12,000 ppm in feed for 78 weeks and held for an additional 26 weeks. Mice were given 0, 11,700, or 23,500 ppm in feed in 104 weeks. The authors concluded that under the conditions shown in this study, there were no convincing evidence that 1-H-benzotriazole was carcinogenic in rats or mice. |
| Purification Methods | 1,2,3-Benzotriazole crystallises from toluene, CHCl3, Me2NCHO or a saturated aqueous solution, and is dried at room temperature or in a vacuum oven at 65o. Losses are less if the material is distilled in a vacuum. CAUTION: may EXPLODE during distillation; necessary precautions must be taken. [Damschroder & Peterson Org Synth Coll Vol III 106 1955, Beilstein 26 III/IV 93.] |
Relevant Products
-
2H-Benzimidazole-2-thione,1,3-dihydro-5-nitro-
CAS:6325-91-3
-
Fluorescent Brightener 351
CAS:54351-85-8