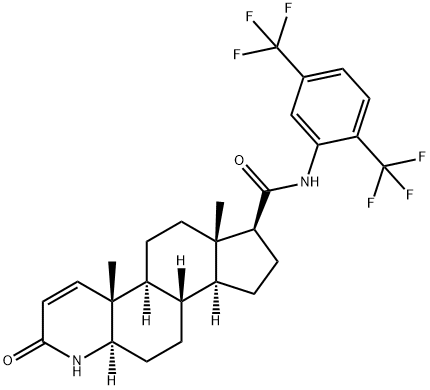
164656-23-9
- Product Name:Dutasteride
- Molecular Formula:C27H30F6N2O2
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 164656-23-9
Molecular Formula: C27H30F6N2O2
Appearance: white crystalline solid
|
164656-23-9 Name |
|
|
Name |
Dutasteride |
|
Synonym |
(1S,2R,7R,10S,11S,14S,15S)-N-[2,5-bis(trifluoroMethyl)phenyl]-2,15-diMethyl-5-oxo-6-azatetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-3-ene-14-carboxaMide;(4aR,4bS,6aS,7S,9aS,9bS,11aR)-N-[2,5-Bis(trifluoroMethyl)phenyl]-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-4a,6a-diMethyl-2-oxo-1H-Indeno[5,4-f]quinoline-7-carboxaMide;(5α,17β)-;(4aR,4bS,6aS,7S,9aS,9bS,11aR)-N-[2,5-Bis(trifluoroMethyl)phenyl]-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-4a,6a-diMethyl-2-oxo-1H-indeno[5,4-f]quinoline-7-carboxaMide-13C6;6β-Hydroxy Dutasteride;4-Azaandrost-1-ene-17-carboxaMide, N-[2,5-bis(trifluoroMethyl)phenyl]-3-oxo-, (5a,17b)-;Avolve;5,6-Dehydro-17&alpha |
|
164656-23-9 Biological Activity |
|
|
Description |
Dutasteride (GG745) is a potent inhibitor of both 5 alpha-reductase isozymes. Dutasteride may possess off-target effects on the androgen receptor (AR) due to its structural similarity to DHT.IC50 Value:Target: 5 alpha-reductasein vitro: Dutasteride inhibited (3)H-T conversion to (3)H-DHT and, as anticipated, inhibited T-induced secretion of PSA and proliferation. However the drug also inhibited DHT-induced PSA secretion and cell proliferation (IC(50) approximately 1 microM). Dutasteride competed for binding the LNCaP cell AR with an IC(50) approximately 1.5 microM. High concentrations of dutasteride (10-50 microM), but not finasteride, in steroid-free medium, resulted in enhanced cell death, possibly by apoptosis [1]. Dutasteride reduces cell viability and cell proliferation in both cell lines tested (androgen-responsive (LNCaP) and androgen-unresponsive (DU145) human prostate cancer (PCa)) [2].in vivo: GG745 has a terminal half-life of approximately 240 hr, and single doses of >10 mg decreased DHT levels significantly more than did single 5-mg doses of finasteride [3]. In placebo treated men without prostate cancer there was an 8.3% median increase in PSA at month 24 compared with -59.5% in those who received dutasteride, using doubled values to correct for dutasteride treatment [4].Toxicity: Dutasteride may affect male fertility and steroid hormone dynamics. Therefore, a 21-day reproduction study was conducted to determine the effects of dutasteride (10, 32 and 100 μg/L) on fish reproduction. Exposure to dutasteride significantly reduced fecundity of fish and affected several aspects of reproductive endocrine functions in both males and females [5].Clinical trial: Bioequivalence Study Of Dutasteride Five 0.1 mg And One 0.5 mg Soft Gelatin Capsules In Healthy Male Volunteers. |
|
Related Catalog |
Signaling Pathways >> Metabolic Enzyme/Protease >> 5 alpha Reductase Research Areas >> Cancer |
|
References |
[1]. Lazier CB, et al. Dutasteride, the dual 5alpha-reductase inhibitor, inhibits androgen action and promotes cell death in the LNCaP prostate cancer cell line. Prostate. 2004 Feb 1;58(2):130-44. [2]. Biancolella M, et al. Effects of dutasteride on the expression of genes related to androgen metabolism and related pathway in human prostate cancer cell lines. Invest New Drugs. 2007 Oct;25(5):491-7. [3]. Bramson HN, et al. Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J Pharmacol Exp Ther. 1997 Sep;282(3):1496-502. [4]. Andriole GL, et al. Clinical usefulness of serum prostate specific antigen for the detection of prostate cancer is preserved in men receiving the dual 5alpha-reductase inhibitor dutasteride. J Urol. 2006 May;175(5):1657-62. [5]. Margiotta-Casaluci L, et al. Mode of action of human pharmaceuticals in fish: the effects of the 5-alpha-reductase inhibitor, dutasteride, on reproduction as a case study. Aquat Toxicol. 2013 Mar 15;128-129:113-23. |
|
164656-23-9 Chemical & Physical Properties |
|
|
Melting point |
242-250ºC |
|
Boiling point |
620.3±55.0 °C at 760 mmHg |
|
Density |
1.3±0.1 g/cm3 |
|
Molecular Formula |
C27H30F6N2O2 |
|
Molecular Weight |
528.530 |
|
Flash Point |
329.0±31.5 °C |
|
PSA |
58.20000 |
|
LogP |
5.61 |
|
Exact Mass |
528.221130 |
|
Vapour Pressure |
0.0±1.8 mmHg at 25°C |
|
Index of Refraction |
1.523 |
|
Storage condition |
-20°C Freezer |
|
Water Solubility |
DMSO: soluble2mg/mL, clear |
|
164656-23-9 Description |
|
Dutasteride is a synthetic 4-azasteroid compound. It is a dual inhibitor of type 1 and 2 isoforms of 5α-reductase unlike finasteride, the first marketed 5α-reductase inhibitor, which only acts on type 2 isozyme. Dutasteride is a 3-fold greater inhibitor of type-2 5α-reductase than finasteride in men and has greater effect on the type-l than on type-2 isozyme. In animal models,dutasteride exhibited superior efficacy and pharmacokinetics compared to finasteride. In patients with benign prostate hyperplasia, administration of dutasteride was shown to dose-dependently decrease serum dihydrotestosterone levels with greater efficacy as compared to finasteride (95% vs 67%). Serum testosterone levels increased with both active drugs, in conjunction with dihydrotestosterone suppression but remained within normal ranges. In long term studies, in men with moderate to severe benign prostate hyperplasia, once daily dutasteride significantly reduced prostate volume, reduced the risk of acute urinary retention and surgery by 57% and improved lower urinary tract symptoms and urinary flow measurements. |
|
164656-23-9 Uses |
|
Dutasteride is a dual inhibitor of 5a-reductase isoenzymes type 1 and 2; structurally related to Finasteride. Dutasteride is used in the treatment of benign prostatic hyperplasia. Dutasteride is associated with a low rate of transient serum aminotransferase elevations, but has yet to be linked to instances of clinically apparent acute liver injury. |
Relevant Products
-
Triethoxyoctylsilane
CAS:2943-75-1