Product Details;
CasNo: 50-28-2
Molecular Formula: C18H24O2
Appearance: white crystalline powder
Fast Delivery High Quality Estradiol Cas No.50-28-2 Best Price
|
50-28-2 Name |
|
|
Name |
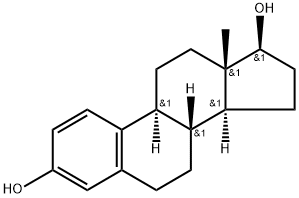
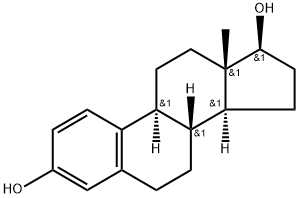
Estradiol |
|
Synonym |
BETA-ESTRADIOL-16,16,17-D3;17BETA-ESTRADIOL-16,16,17-D3;β-Estradiol, 99% (dry wt.), ca 3% water;1,3,5-Estratriene-3,17beta-diol;17beta-Estradiol;3,17beta-Dihydroxy-1,3,5(10)-estratriene;Dihydrofolliculin;Estradiol |
|
50-28-2 Chemical & Physical Properties |
|
|
Melting point |
173ºC |
|
Boiling point |
445.9±45.0 °C at 760 mmHg |
|
Density |
1.2±0.1 g/cm3 |
|
Molecular Formula |
C18H24O2 |
|
Molecular Weight |
272.382 |
|
Flash Point |
209.6±23.3 °C |
|
PSA |
40.46000 |
|
LogP |
4.13 |
|
Exact Mass |
272.177643 |
|
Vapour Pressure |
0.0±1.1 mmHg at 25°C |
|
Index of Refraction |
1.599 |
|
Storage condition |
2-8°C |
|
Stability |
Stable. Incompatible with strong oxidizing agents. |
|
solubility |
Practically insoluble in water, soluble in acetone, sparingly soluble in ethanol (96 per cent), slightly soluble in methylene chloride. |
|
storage temp. |
room temp |
|
50-28-2 Description |
|
β-Estradiol is an endogenous estrogenic hormone receptor (ER) agonist (Ki values are 0.12 and 0.13 nM for ERα and ERβ respectively). Also high affinity ligand at membrane estrogen GPR30 receptors. β-Estradiol is an activator of PI 3-kinase. Estradiol (17β-estradiol, β-Estradiol, E2, 17β-Oestradiol) is a human sex hormone and steroid, and the primary female sex hormone. Estradiol upregulates IL-6 expression through the estrogen receptor β (ERβ) pathway. |
|
50-28-2 Uses |
|
|
50-28-2 Safety Profile |
|
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. A promoter. Human reproductive effects by ingestion: ferthty effects. Experimental reproductive effects. Human mutation data reported. A steroid hormone much used in medicine. When heated to decomposition it emits acrid smoke and irritating fumes. |
Estradiol Relevant articles
Neuroprotection by estradiol.
Luis Miguel Garcia-Segura a,Iigo Azcoitia b,Lydia L. DonCarlos c
, Progress in Neurobiology, 2001
Recent basic science studies show that not only does exogenous estradiol decrease the response to various forms of insult, but the brain itself upregulates both estrogen synthesis and estrogen receptor expression at sites of injury.
|
50-28-2 Purification Methods |
|
17-Estradiol (previously known as -estradiol) is purified by chromatography on SiO2 (toluene/EtOAc 4:1) and recrystallised from CHCl3/hexane or 80% EtOH. It is stable in air, is insoluble in H2O, and is precipitated by digitonin. The UV has max at 225 and 280 nm. The diacetate [3434-88-6] has m 97-98o and forms leaflets from aqueous EtOH. The 3-benzoate crystallises from aqueous MeOH withm 193o and [] D 25 +58o to 63o (c 1, dioxane). [Meischer & Scholz Helv Chim Acta 20 263, 1237 1937, Biochem J 32 1273 1938, Oppolzer & Roberts Helv Chim Acta 63 1703 1980, Inhoffen & Zühlsdorff Chem Ber 7 4 1914 1941, Beilstein 6 IV 6611.] |
Relevant Products
-
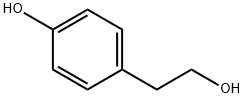
4-Hydroxyphenethyl alcoholCAS NO.: 501-94-0
CAS:501-94-0
-
Estradiol
CAS:50-28-2
-
Estradiol valerate
CAS:979-32-8