881674-56-2
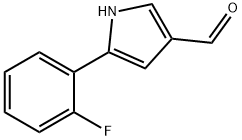
- Product Name:1H-Pyrrole-3-carboxaldehyde, 5-(2-fluorophenyl)-
- Molecular Formula:C11H8FNO
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 881674-56-2
Molecular Formula: C11H8FNO
881674-56-2 Properties
- Molecular Formula:C11H8FNO
- Molecular Weight:189.189
- PKA:14.77±0.50(Predicted)
- PSA:32.86000
- LogP:2.63330
881674-56-2 Usage
Chemical Properties
Light orange to Yellow to Green powder to crystal.
Uses
5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde acts as a reagent in the synthetic preparation of novel pyrrole derivatives as potassium-competitive acid blocker (P-CAB).
Application
5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde is a potassium ion-competitive acid blocker used in therapy Acid-related diseases, gastric ulcer, duodenal ulcer, reflux esophagitis, etc.
Preparation
Synthesis of 5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde: Using pyrrole as raw material, after being protected by N-alkylation of triisopropylsilyl chloride, it is then reacted with Vilsmeier reagent to obtain 1H-Pyrrole-3-carbaldehyde, which is then purified by N-bromosuccinimide (NBS) bromination and 2-fluorophenylboronic acid for Suzuki coupling reaction to obtain crude 5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde, which was purified with toluene. The total yield was 35.5%.
881674-56-2 Relevant articles
Preparation method of fumaric acid Vonoprazan intermediate
-
Paragraph 0061-0085, (2021/07/28)
The invention relates to a preparation method of a fumaric acid Vonoprazan intermediate. In order to solve the problems that in the reaction process, many byproducts are produced and the product is easy to degrade, in the post-treatment process, the pH va
Preparation method 5 - (2 - fluorophenyl) - 1H - pyrrole -3 - formaldehyde
-
Paragraph 0079-0100, (2021/10/20)
The invention provides a preparation method of 5 - (2 - fluorophenyl) - 1H - pyrrole -3 - formaldehyde, which comprises the following steps of A: 2 - chlorine -5 - (2 - fluorophenyl) - 1H - pyrrole -3 - carbonitrile in a solvent. In the base and palladium
Synthesis method of 5-(2-fluorophenyl)-1H-pyrrole-3-formaldehyde
-
Paragraph 0023; 0025; 0035-0036, (2021/01/20)
The invention discloses a synthetic method of 5-(2-fluorophenyl)-1H-pyrrole-3-formaldehyde, and belongs to the field of organic synthesis. The preparation method comprises the following steps: (1) reacting 2-fluoro acetophenone with a bromination reagent
Preparation method 5 - (2 - fluorophenyl) - 1H - pyrrole -3 - formaldehyde
-
Paragraph 0072-0089, (2021/11/19)
The invention belongs to the technical field of pharmaceutical chemicals, and particularly relates to a preparation method of 5 - (2 - fluorophenyl) - 1H - pyrrole -3 - formaldehyde. The preparation method of 5 - (2 - fluorophenyl) - 1H - pyrrole -3 - for
881674-56-2 Process route
-

- 1240948-77-9
5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile

-

- 881674-56-2
5-(2-fluorophenyl)-1H-pyrrole-3-carboxaldehyde
| Conditions | Yield |
|---|---|
|
With hydrogen; acetic acid; In tetrahydrofuran; water; at 20 - 30 ℃; for 5h; Inert atmosphere;
|
90.54% |
|
With hydrogen; acetic acid; In tetrahydrofuran; water; at 15 - 25 ℃; for 4h; Reagent/catalyst; Temperature;
|
84.4% |
|
With pyridine; hydrogen; acetic acid; In water; at 30 ℃; for 20h; Temperature; Reagent/catalyst; Industrial scale;
|
84.9% |
|
5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile; With hydrogen; acetic acid; Raney-nickel; In tetrahydrofuran; water; at 15 - 25 ℃; for 3h;
With water; sodium hydroxide; In tetrahydrofuran; ethyl acetate; at 10 - 35 ℃; pH=7 - 8;
With hydrogenchloride; water; In tetrahydrofuran; ethyl acetate; at 15 - 25 ℃; pH=3 - 3.5;
|
78% |
|
5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile; With hydrogen; acetic acid; In tetrahydrofuran; at 20 ℃; for 3h;
With water; sodium hydroxide; at 20 ℃; pH=7 - 8;
|
76.9% |
|
With formic acid; water; hydrogen; In tetrahydrofuran; for 2.5h; under 750.075 Torr;
|
51.5% |
|
With hydrogen; acetic acid; In tetrahydrofuran; water; at 20 ℃; for 2h;
|
42% |
-

- 881674-58-4
(5-(2-fluorophenyl)-1H-pyrrol-3-yl)methanol

-

- 881674-56-2
5-(2-fluorophenyl)-1H-pyrrole-3-carboxaldehyde
| Conditions | Yield |
|---|---|
|
With sodium bromate; sulfuric acid; bromine; sodium bromide; In dichloromethane; water; butan-1-ol; at 0 - 5 ℃; for 3.5h; Solvent;
|
95.1% |
|
With [bis(acetoxy)iodo]benzene; In dichloromethane; at 20 ℃; for 12h;
|
92% |
|
With tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide; In acetonitrile; at 20 ℃; for 1.5h;
|
60% |
|
With 4-methylmorpholine N-oxide; tetrapropylammonium perruthennate; In acetonitrile; at 20 ℃; for 1.5h;
|
|
|
With tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide; In acetonitrile; at 20 ℃; for 1.5h; Molecular sieve;
|
881674-56-2 Upstream products
-
881674-58-4

(5-(2-fluorophenyl)-1H-pyrrol-3-yl)methanol
-
114615-82-6

tetrapropylammonium perruthennate
-
881674-06-2

5-(2-fluorophenyl)-1H-pyrrole-3-carboxylic acid ethyl ester
-
1240948-72-4

2-chloro-5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile
881674-56-2 Downstream products
-
928324-56-5

4-chloro-5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde
-
928324-57-6

4-fluoro-5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde
-
928324-91-8

5-(2-fluorophenyl)-4-iodo-1H-pyrrole-3-carbaldehyde
-
881677-09-4

5-(2-fluorophenyl)-1-[(2-fluorophenyl)sulfonyl]-1H-pyrrole-3-carbaldehyde
Relevant Products
-
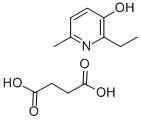
Butanedioic acid 2-ethyl-6-methyl-3-pyridinol (1:1)
CAS:127464-43-1
-
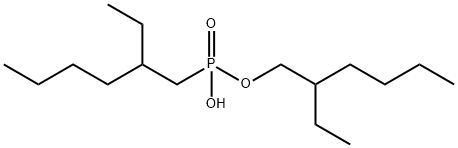
Phosphonic acid,P-(2-ethylhexyl)-, mono(2-ethylhexyl) ester
CAS:14802-03-0