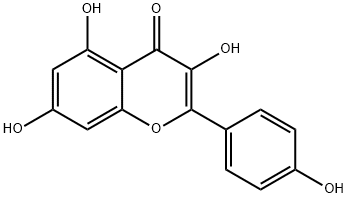
520-18-3
- Product Name:4H-1-Benzopyran-4-one,3,5,7-trihydroxy-2-(4-hydroxyphenyl)-
- Molecular Formula:C15H10O6
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 520-18-3
Molecular Formula: C15H10O6
Appearance: Yellow solid
520-18-3 Properties
- Molecular Formula:C15H10O6
- Molecular Weight:286.241
- Appearance/Colour:Yellow solid
- Vapor Pressure:6.38E-16mmHg at 25°C
- Melting Point:276 °C
- Refractive Index:1.767
- Boiling Point:582.1 °C at 760 mmHg
- PKA:6.34±0.40(Predicted)
- Flash Point:226.1 °C
- PSA:111.13000
- Density:1.688 g/cm3
- LogP:2.28240
520-18-3 Usage
Pharmacological effects
Kaempferol (3,5,7‐trihydroxy‐2‐[4‐hydroxyphenyl]‐4H‐1‐benzopyran‐4‐one) is a yellow bioactive flavonoid, which is present inmany edible plants such as tea, cabbage, broccoli, endive, kale, beans, tomato, strawberries, leek, and grapes. It has a significant role in reducing cancer and can act as a therapeutic agent in the treatment of diseases and ailments such as diabetes, obesity, cardiovascular diseases, oxidative stress, asthma, and microbial contamination disorders. Its efficacy, a broad range of activity, and low toxicity compared with other examined compounds, make it an attractive chemical in the fight against diseases (including cancer).
Mechanism of action
Kaempferol acts through different mechanisms: It induces apoptosis (HeLa cervical cancer cells), decreases cell viability (G2/M phase), downregulates phosphoinositide 3‐kinase (PI3K)/AKT (protein kinase B) and human T‐cell leukemia/lymphoma virus‐I (HTLV-I) signaling pathways, suppresses protein expression of epithelial‐mesenchymal transition (EMT)‐related markers including N‐cadherin, E‐cadherin, Slug, and Snail, and metastasis‐related markers such as matrix metallopeptidase 2 (MMP-2).
Bioactivity
As an anti‐oxidant, kaempferol counteracts production of superoxide ions and lowers the formation of reactive oxygen and nitrogen species. It also scavenges Fenton‐generated hydroxyl radical, peroxynitrite, and hydroxyl radicals. Furthermore, kaempferol suppresses the activity of xanthine oxidase and enhances the activities of catalase, heme oxygenase‐1, and superoxide dismutase.
Chemical Properties
Yellow Solid
Uses
antidepressant, inhibits fatty acid amide hydrolase
Uses
Kaempferol, is used as an inhibitor of Fatty Acid Synthase, Cox-1 activity, and Topo I. It also Induces caspase-9-mediated apoptosis in a variety of cancer cell lines via downregulation of polo-like kinase 1 (PLK1) expression. Exhibits antioxidant activity and attenuates osteoclastic bone reabsorption in vitro. Also blocks EGF-induced histone H3Ser10 phosphorylation in mouse epidermal JB6 C141 cells.
Uses
Chromogenic reagent for antimony in the low ppm range and for gallium and indium in the sub-ppm range.
General Description
Kaempferol is a polyphenolic antioxidant abundantly present in vegetables and fruits. It has a diphenylpropane structure. In many plants, it is present as a glycosidic form namely, kaempferol-3-O-glucoside.
Biological Activity
Naturally occurring flavonoid found in Gingko biloba and red wines that activates the mitochondrial Ca 2+ uniporter (EC 50 = 7 μ M). Induces caspase-9-mediated apoptosis in a variety of cancer cell lines via downregulation of polo-like kinase 1 (PLK1) expression. Exhibits antioxidant activity and attenuates osteoclastic bone reabsorption in vitro .
Biochem/physiol Actions
Potent inhibitor of osteoclastic bone resorption. The effect is believed to be attributable to both the antioxidant and estrogenic activities of kaempferol.
Anticancer Research
Kaempferol is one of the secondary metabolites found in some plants, plant-derivedfoods, and traditional medicines. It is a flavonoid compound obtained from someedible plants including grapes, tea, strawberries, broccoli, tomato, cabbage, leek,kale, endive, and beans. It inhibits growth and migration of pancreatic cancer cellsby acting on proto-oncogene tyrosine kinase (Src), ERK1/2, and AKT pathways(Singh et al. 2016a). It is being investigated in pancreatic and lung cancers toevaluate its antiangiogenic, anticancer, and radical scavenging activities. It showsmoderate cytostatic activity in PC3, HeLa, and K562 human cancer cells. It isidentified as aryl hydrocarbon receptor antagonist and acts against ABCG2 (ATP-bindingcassette subfamily G member 2)-mediated multidrug resistance bypreventing the ABCG2 upregulation in esophageal carcinoma. It induces theapoptosis of ovarian cancer cell by activating p53 in intrinsic pathway mechanism.It is an inhibitor of breast cancer resistance protein (BCRP), quinine reductase-2,and a substrate of BCRP (Calderon-Montano et al. 2011; Wang et al. 2012).
InChI:InChI=1/C15H10O6/c16-7-1-2-9(10(18)3-7)13-6-12(20)15-11(19)4-8(17)5-14(15)21-13/h1-6,16-19H
520-18-3 Relevant articles
Flavonoids as effective protectors of urease from ultrasonic inactivation in solutions
Tarun,Kurchenko,Metelitza
, p. 352 - 359 (2006)
Inactivation of soybean urease in aqueous solution at pH 5.4, 36°C, and high-frequency sonication (2.64 MHz, 1.0 W/cm2) is substantially reduced in the presence of seven structurally different flavonoids. A comparative kinetic study of the effect of these flavonoids on the effective first-order rate constants that characterize the total (thermal and ultrasonic) inactivation k i , thermal inactivation k*i, and ultrasonic inactivation k i (US) of 25 nM enzyme solution was carried out. The dependences of the three inactivation rate constants of the urease on the concentrations of flavonoids within the range from 10-11 to 10-4 M were obtained. The following order of the efficiency of the flavonoids used in respect of the urease protection from ultrasonic inactivation was found: astragalin > silybin > naringin > hesperidin > quercetin > kaempferol > morin. The results confirm a significant role in the inactivation of the urease of HO. and HO 2 . free radicals, which are formed in the ultrasonic cavitation field. Pleiades Publishing, Inc., 2006.
Bioactive compounds of the flora of belarus. 3.* Gymnocarpium dryopteris, a kaempferol source
Kovganko,Kashkan,Krivenok
, p. 16 - 18 (2004)
Kaempferol (2) was isolated from the aerial part of the oak fern Gymnocarpium dryopteris by extraction and subsequent acid hydrolysis of the glycoside mixture.
KAEMPFEROL DERIVATIVES FROM THE FRUIT OF THE JAPANESE PAGODA TREE
Akhmedkhodzhaeva, N. M.,Svechnikova, A. N.
, p. 116 - 117 (1983)
-
-
Zakharov et al.
, (1971)
-
-
Gumenyuk et al.
, (1971)
-
-
Dungerdorzh,Petrenko
, (1972)
-
Tannins, flavonol sulfonates, and a norlignan from Phyllanthus virgatus
Huang, Yu-Lin,Chen, Chien-Chih,Hsu, Feng-Lin,Chen, Chieh-Fu
, p. 1194 - 1197 (1998)
Investigation of the constituents of Phyllanthus virgatus has led to the isolation of five new compounds, including a norlignan, 2-(3,4- methylenedioxybenzyl)-4-(3,4-methylenedioxyphenyl)-3-butyne-1,2-diol named virgatyne (1); a hydrolyzable tannin, virganin (2); and three flavonoid sulfonates, galangin-8-sulfonate (4), galangin-3-O-β-D-glucoside-8- sulfonate (5), and kaempferol-8-sulfonate (6). Their structures were established by spectral and chemical methods.
-
Chumbalov et al.
, (1972)
-
FLAVONOIDS OF THE FLOWERS OF Cyclachaena xanthifolia
Kurkin, V. A.,Zapesochnaya, G. G.,Krivenchuk, P. E.,Yurkenik, A. Yu.,Artamonova, L. P.
, p. 366 - 367 (1984)
-
-
Medveda et al.
, (1972)
-
A new acylated flavonol glycoside from the aerial parts of Cardamine tangutorum
Feng, Wei-Sheng,Zhang, Qiu-Bo,Zheng, Xiao-Ke,Chen, Hui,Zhang, Yan-Li,Zhang, Chun-Lei
, p. 805 - 810 (2012)
A new acylated flavonol glycoside, kaempferol-3-O-β-d-(2- feruloylglucopyranosyl) (1 → 6)-[β-d-glucopyranosyl(1 → 2)]-β-d-glucopyranoside, named tangutorumoside A (1), together with 12 known compounds, was isolated from 50% acetone extract of Cardamine tangutorum. Their structures were elucidated by NMR and MS experiments. In addition, compound 1 could promote the proliferation of splenic lymphocytes and thymic lymphocytes with ConA in vitro.
FLAVONOIDS OF Astragalus lagurus
Guzhva, N. N.,Kazakov, A. L.,Dzhumyrko, S. F.,Sarkisov, L. S.
, p. 627 - 628 (1984)
-
-
Roshchin
, (1977)
-
-
Sosa,Percheron
, p. 441,445 (1970)
-
-
Samokish,Shinkurenko
, (1969)
-
-
Zurabishvili,Kemertelidze
, (1971)
-
Anti-complement activity of tiliroside from the flower buds of Magnolia fargesii
Jung, Keun Young,Oh, Sei Ryang,Park, Si-Hyung,Lee, Im Seon,Ahn, Kyung Seop,Lee, Jung Joon,Lee, Hyeong-Kyu
, p. 1077 - 1078 (1998)
As part of the search for anticomplementary active components from natural products, the anticomplementary properties of methanolic extracts from the flower buds of Magnolia fargesii have been investigated. Bioassay- guided chromatographic Separation of the active constituents led to the isolation of compound 1, whose structure was identified by spectroscopic methods to be kaempferol 3-O-β-D-(6-O-coumaroyl)glucopyranoside (tiliroside). Tiliroside showed very potent anti-complement activity (IC50=5.4x 10-5 M) on the classical pathway of the complement system, even higher than rosmarinic acid, which is a well-known inhibitor against the complement system. On the other hand, the hydrolysates of tiliroside, kaempferol, astragalin and p-coumaric acid showed very weak activity on this system.
Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol
Wang, Jingqiu,Fang, Xianying,Ge, Lin,Cao, Fuliang,Zhao, Linguo,Wang, Zhenzhong,Xiao, Wei
, (2018)
Kaempferol (kae) and its glycosides are widely distributed in nature and show multiple bio-activities, yet few reports have compared them. In this paper, we report the antitumor, antioxidant and anti-inflammatory activity differences of kae, kae-7-O-gluco
-
Betkhi Tkhuan',Blinova
, (1974)
-
Isolation, characterization, complete structural assignment, and anticancer activities of the methoxylated flavonoids from rhamnus disperma roots
Abd El-Wahab, Mohammed F.,Abdalla, Ashraf N.,Mohammed, Abd El-Salam I.,Mohammed, Hamdoon A.,Ragab, Ehab A.,Shaheen, Usama
, (2021/10/01)
Different chromatographic methods including reversed-phase HPLC led to the isolation and purification of three O-methylated flavonoids; 5,4’-dihydroxy-3,6,7-tri-O-methyl flavone (penduletin) (1), 5,3’-dihydroxy-3,6,7,4’,5’-penta-O-methyl flavone (2), and 5-hydroxy-3,6,7,3’,4’,5’-hexa-O-methyl flavone (3) from Rhamnus disperma roots. Additionlly, four flavonoid glycosides; kampferol 7-O-α-L-rhamnopyranoside (4), isorhamnetin-3-O-β-D-glucopyranoside (5), quercetin 7-O-α-L-rhamnopyranoside (6), and kampferol 3, 7-di-O-α-L-rhamnopyranoside (7) along with benzyl-O-β-D-glucopyranoside (8) were successfully isolated. Complete structure characterization of these compounds was assigned based on NMR spectroscopic data, MS analyses, and comparison with the literature. The O-methyl protons and carbons of the three O-methylated flavonoids (1–3) were unambiguously assigned based on 2D NMR data. The occurrence of compounds 1, 4, 5, and 8 in Rhamnus disperma is was reported here for the first time. Compound 3 was acetylated at 5-OH position to give 5-O-acetyl-3,6,7,3’,4’,5’-hexa-O-methyl flavone (9). Compound 1 exhibited the highest cytotoxic activity against MCF 7, A2780, and HT29 cancer cell lines with IC50 values at 2.17 μM, 0.53 μM, and 2.16 μM, respectively, and was 2–9 folds more selective against tested cancer cell lines compared to the normal human fetal lung fibroblasts (MRC5). It also doubled MCF 7 apoptotic populations and caused G1 cell cycle arrest. The acetylated compound 9 exhibited cytotoxic activity against MCF 7 and HT29 cancer cell lines with IC50 values at 2.19 μM and 3.18 μM, respectively, and was 6–8 folds more cytotoxic to tested cancer cell lines compared to the MRC5 cells.
Flavonol Glycosides from Leaves of Allium microdictyon
Olennikov
, p. 1035 - 1039 (2020/11/03)
The composition of flavonoids from leaves of Allium microdictyon Prokh. (Amaryllidaceae) was studied for the first time and included 14 compounds including two new flavonol glycosides 1 and 2. UV, IR, and NMR spectroscopic and mass spectrometric data dete
Synthesis and Biological Evaluation of 4-Substituted Kaempfer-3-ols
Kim, Sugyeom,Lannigan, Deborah A.,Li, Yu,Lin, Lin,O'Doherty, George A.,Sayasith, Peyton R.,Tarr, Ariel T.,Wright, Eric B.,Yasmin, Sharia
, p. 4279 - 4288 (2020/04/09)
The synthesis of two series of five kaempfer-3-ols was described. The first set all have a C-3 hydroxyl group and the second has a carboxymethoxy ether at the C-3 position. Both series have variable substitution at the C-4 position (i.e., OH, Cl, F, H, OMe). Both kaempferols and carboxymethoxy ethers were evaluated for their ability to inhibit ribosomal s6 kinase (RSK) activity and cancer cell proliferation.
Flavonoid glycosides from seeds of Hippophae rhamnoides subsp. Sinensis with α-glucosidase inhibition activity
Li, Rui,Wang, Qing,Zhao, Menghao,Yang, Peiming,Hu, Xiao,Ouyang, Danwei
, (2019/07/04)
Hippophae rhamnoides subsp. Sinensis is a famous traditional medicinal plant in Tibet and Mongolia of China. Three novel flavonoid glycosides and ten known analogues were obtained from the seeds of H. rhamnoides. The structures of new compounds were elucidated by spectroscopics, chemical methods as well as literature data. In vitro assay, compounds 5–9, kaempferol and 70% ethanolic elution fraction showed prominent α-glucosidase inhibitory activities with IC50 values ranging from 8.30 to 112.11 μM, better than that of the positive control, acarbose, whose IC50 value was 1727.07 μM.
520-18-3 Process route
-

- 1449692-21-0
kaempferol-3-O-α-D-xylopyranosyl-(2a→1b)-2a-O-α-D-xylopyranosyl-(2b→1c)-2b-O-α-D-xylopyranosyl-(2c→1d)-2c-O-α-D-xylopyranosyl-2d-hexadecanoate

-

- 58-86-6
D-xylose

-

- 520-18-3
kaempferol

-

- 57-10-3
1-hexadecylcarboxylic acid
| Conditions | Yield |
|---|---|
|
kaempferol-3-O-α-D-xylopyranosyl-(2a→1b)-2a-O-α-D-xylopyranosyl-(2b→1c)-2b-O-α-D-xylopyranosyl-(2c→1d)-2c-O-α-D-xylopyranosyl-2d-hexadecanoate; With hydrogenchloride; In ethanol; water; at 70 - 80 ℃;
With hydrogenchloride; In ethanol; water;
|
-

-
C27H29O16(1-)*Na(1+)

-

- 492-61-5
β-D-glucose

-

- 520-18-3
kaempferol
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; at 90 ℃; for 5h;
|
520-18-3 Upstream products
-
480-20-6

Aromadendrin
-
480-10-4

astragalin
-
22688-78-4

kaempferol 3-O-β-D-glucuronide
-
480-10-4

astragalin
520-18-3 Downstream products
-
15486-34-7

5-hydroxy-3,4',7-trimethoxyflavone
-
134-04-3

pelargonidin chloride
-
724434-08-6

dihydrokaempferol
-
480-10-4

astragalin
Relevant Products
-
4-Hydroxyphenethyl alcoholCAS NO.: 501-94-0
CAS:501-94-0
-
Melanotan II
CAS:121062-08-6
-
2-Butyn-1-ol
CAS:764-01-2