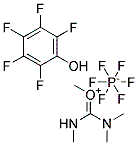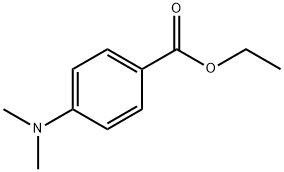
10287-53-3
- Product Name:Ethyl 4-dimethylaminobenzoate
- Molecular Formula:C11H15NO2
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 10287-53-3
Molecular Formula: C11H15NO2
Appearance: White crystal powder
10287-53-3 Properties
- Molecular Formula:C11H15NO2
- Molecular Weight:193.246
- Appearance/Colour:White crystal powder
- Vapor Pressure:0mmHg at 25°C
- Melting Point:62-65 °C
- Refractive Index:1.5080 (estimate)
- Boiling Point:296.5 °C at 760 mmHg
- PKA:2.56±0.12(Predicted)
- Flash Point:115 °C
- PSA:29.54000
- Density:1.061 g/cm3
- LogP:1.92930
10287-53-3 Usage
Chemical Properties
white crystalline powder
Uses
Ethyl 4-dimethylaminobenzoate is employed as a photoinititator in cell encapsulation applications. It is also useful for organic synthesis. Further, it is used in UV-curing coatings and inks for the initiation of photo polymerization of unsaturated prepolymers. In addition to this, it acts as a active pharmaceutical ingredient intermediate.
Uses
Et-PABA is used as a co-initiator with camphorquinone (CQ) to form a self-adhesive composite for the fabrication of photosensitizers in dental materials.
Definition
ChEBI: A benzoate ester that is ethyl benzoate substituted by a dimethylamino group at position 4.
Flammability and Explosibility
Notclassified
InChI:InChI=1/C11H15NO2/c1-4-8-7-9(12(2)3)5-6-10(8)11(13)14/h5-7H,4H2,1-3H3,(H,13,14)/p-1
10287-53-3 Relevant articles
Combretastatin linked 1,3,4-oxadiazole conjugates as a Potent Tubulin Polymerization inhibitors
Kamal, Ahmed,Srikanth,Vishnuvardhan,Kumar, G. Bharath,Suresh Babu, Korrapati,Hussaini, S.M. Ali,Kapure, Jeevak Sopanrao,Alarifi, Abdullah
, p. 126 - 136 (2016)
A new class of combretastatin linked 1,3,4-oxadiazoles were designed, synthesized and screened for their cytotoxic activity against five human cancer cell lines such as HeLa, DU-145, A549, MDA-MB-231 and B16. These compounds showed significant cytotoxicity with IC50 values in the range 0.118-54.32 μM. Conjugate 5m displayed potent antiproliferative activity against DU-145 cell line. Flow cytometric analysis revealed that these compounds arrested the cell cycle in G2/M phase. Moreover, the tubulin polymerization assay and immunofluorescence analysis indicate that 5m exhibits potent inhibitory effect on the tubulin assembly. Further, DNA fragmentation and Hoecst staining assays confirm that 5m induces apoptosis. Molecular docking studies and competitive binding assay indicated that 5m effectively bind at the colchicine binding site of the tubulin.
Alkyl formates as reagents for reductive amination of carbonyl compounds
Afanasyev, Oleg I.,Cherkashchenko, Ilia,Kuznetsov, Anton,Kliuev, Fedor,Semenov, Sergey,Chusova, Olga,Denisov, Gleb,Chusov, Denis
, p. 112 - 113 (2020)
Alkyl formates in the presence of basic additives can serve as a reagent in the direct reductive amination of carbonyl compounds. The developed procedure can be applied to various aldehydes and ketones with electron donating and electron withdrawing groups.
The dual role of ionic liquid BmimBF4, precursor of N-heterocyclic carbene and solvent, in the oxidative esterification of aldehydes
Chiarotto, Isabella,Feroci, Marta,Sotgiu, Giovanni,Inesi, Achille
, p. 8088 - 8095 (2013)
Room temperature ionic liquid BmimBF4 (1-butyl-3- methylimidazolium tetrafluoroborate) has been utilized in the N-heterocyclic carbene-catalyzed oxidation of aldehydes to yield esters. In the presence of MnO2 as oxidant and of DBU and caesium carbonate as bases, aromatic, heteroaromatic and aliphatic esters have been isolated in good to excellent yields. The recyclability of the used ionic liquid along with the excess of inorganic reagents has been proved. The simple and cheap BmimBF4 ionic liquid played the dual role of precatalyst and solvent. This is the first time that such a reaction has been carried out with an ionic liquid as solvent.
Betaine Catalysis for Hierarchical Reduction of CO2 with Amines and Hydrosilane To Form Formamides, Aminals, and Methylamines
Liu, Xiao-Fang,Li, Xiao-Ya,Qiao, Chang,Fu, Hong-Chen,He, Liang-Nian
, p. 7425 - 7429 (2017)
An efficient, sustainable organocatalyst, glycine betaine, was developed for the reductive functionalization of CO2 with amines and diphenylsilane. Methylamines and formamides were obtained in high yield by tuning the CO2 pressure and reaction temperature. Based on identification of the key intermediate, that is, the aminal, an alternative mechanism for methylation involving the C0 silyl acetal and aminal is proposed. Furthermore, reducing the CO2 amount afforded aminals with high yield and selectivity. Therefore, betaine catalysis affords products with a diversified energy content that is, formamides, aminals and methylamines, by hierarchical two-, four- and six-electron reduction, respectively, of CO2 coupled with C?N bond formation.
Alcohol promoted N -methylation of anilines with CO2/H2over a cobalt catalyst under mild conditions
Han, Buxing,Ke, Zhengang,Li, Ruipeng,Liu, Zhimin,Tang, Minhao,Wang, Huan,Zeng, Wei,Zhao, Yanfei
, p. 9147 - 9153 (2021/11/30)
N-Methylation of amines with CO2/H2 to N-methylamines over non-noble metal catalysts is very interesting but remains challenging. Herein, we present an alcohol (e.g., ethanol) promoted strategy for the N-methylation of anilines with CO2/H2 with high efficiency under mild conditions (e.g., 125 °C), which is achieved over a cobalt catalytic system composed of Co(OAc)2·4H2O, triphos and Sn(OTf)2. This catalytic system has a broad substrate scope and is tolerant toward a wide range of anilines and N-methyl anilines, and a series of N,N-dimethyl anilines were obtained in high yields. Mechanism investigation indicates that the alcohol solvent shifts the equilibrium of CO2 hydrogenation by forming an alkyl formate, which further reacts with the amine to produce N-formamide, and Sn(OTf)2 promotes the deoxygenative hydrogenation of N-formamides to afford N-methylamines. This is the first example of the N-methylation of amines with CO2/H2 over a cobalt catalytic system, which shows comparable performance to the reported Ru catalysts and may have promising applications.
Simple RuCl3-catalyzed N-Methylation of Amines and Transfer Hydrogenation of Nitroarenes using Methanol
Sarki, Naina,Goyal, Vishakha,Tyagi, Nitin Kumar,Puttaswamy,Narani, Anand,Ray, Anjan,Natte, Kishore
, p. 1722 - 1729 (2021/04/19)
Methanol is a potential hydrogen source and C1 synthon, which finds interesting applications in both chemical synthesis and energy technologies. The effective utilization of this simple alcohol in organic synthesis is of central importance and attracts scientific interest. Herein, we report a clean and cost-competitive method with the use of methanol as both C1 synthon and H2 source for selective N-methylation of amines by employing relatively cheap RuCl3.xH2O as a ligand-free catalyst. This readily available catalyst tolerates various amines comprising electron-deficient and electron-donating groups and allows them to transform into corresponding N-methylated products in moderate to excellent yields. In addition, few marketed pharmaceutical agents (e. g., venlafaxine and imipramine) were also successfully synthesized via late-stage functionalization from readily available feedstock chemicals, highlighting synthetic value of this advanced N-methylation reaction. Using this platform, we also attempted tandem reactions with selected nitroarenes to convert them into corresponding N-methylated amines using MeOH under H2-free conditions including transfer hydrogenation of nitroarenes-to-anilines and prepared drug molecules (e. g., benzocaine and butamben) as well as key pharmaceutical intermediates. We further enable one-shot selective and green syntheses of 1-methylbenzimidazole using ortho-phenylenediamine (OPDA) and methanol as coupling partners.
Design, synthesis, in vitro and in vivo evaluation against MRSA and molecular docking studies of novel pleuromutilin derivatives bearing 1, 3, 4-oxadiazole linker
Liu, Jie,Zhang, Guang-Yu,Zhang, Zhe,Li, Bo,Chai, Fei,Wang, Qi,Zhou, Zi-Dan,Xu, Ling-Ling,Wang, Shou-Kai,Jin, Zhen,Tang, You-Zhi
, (2021/05/17)
A class of pleuromutilin derivatives containing 1, 3, 4-oxadiazole were designed and synthesized as potential antibacterial agents against Methicillin-resistant staphylococcus aureus (MRSA). The ultrasound-assisted reaction was proposed as a green chemistry method to synthesize 1, 3, 4-oxadiazole derivatives (intermediates 85–110). Among these pleuromutilin derivatives, compound 133 was found to be the strongest antibacterial derivative against MRSA (MIC = 0.125 μg/mL). Furthermore, the result of the time-kill curves displayed that compound 133 could inhibit the growth of MRSA in vitro quickly (- 4.36 log10 CFU/mL reduction). Then, compound 133 (- 1.82 log10 CFU/mL) displayed superior in vivo antibacterial efficacy than tiamulin (- 0.82 log10 CFU/mL) in reducing MRSA load in mice thigh model. Besides, compound 133 exhibited low cytotoxicity to RAW 264.7 cells. Molecular docking studies revealed that compound 133 was successfully localized in the binding pocket of 50S ribosomal subunit (ΔGb = -10.50 kcal/mol). The results indicated that these pleuromutilin derivatives containing 1, 3, 4-oxadiazole might be further developed into novel antibiotics against MRSA.
Metal-Free Deoxygenation of Amine N-Oxides: Synthetic and Mechanistic Studies
Lecroq, William,Schleinitz, Jules,Billoue, Mallaury,Perfetto, Anna,Gaumont, Annie-Claude,Lalevée, Jacques,Ciofini, Ilaria,Grimaud, Laurence,Lakhdar, Sami
, p. 1237 - 1242 (2021/06/01)
We report herein an unprecedented combination of light and P(III)/P(V) redox cycling for the efficient deoxygenation of aromatic amine N-oxides. Moreover, we discovered that a large variety of aliphatic amine N-oxides can easily be deoxygenated by using only phenylsilane. These practically simple approaches proceed well under metal-free conditions, tolerate many functionalities and are highly chemoselective. Combined experimental and computational studies enabled a deep understanding of factors controlling the reactivity of both aromatic and aliphatic amine N-oxides.
10287-53-3 Process route
-

-
C36H60O30*C11H15NO2

-

- 10287-53-3
ethyl p-dimethyaminolbenzoate

-

- 10016-20-3
alpha cyclodextrin
| Conditions | Yield |
|---|---|
|
In water; Equilibrium constant;
|
-

-
C42H70O35*C11H15NO2

-

- 10287-53-3
ethyl p-dimethyaminolbenzoate

-

- 7585-39-9
β‐cyclodextrin
| Conditions | Yield |
|---|---|
|
In water; Equilibrium constant;
|
10287-53-3 Upstream products
-
64-17-5

ethanol
-
619-84-1

p-N,N-dimethylaminobenzoic acid
-
94-09-7

p-aminoethylbenzoate
-
4755-50-4

4-(dimethylamino)benzoyl chloride
10287-53-3 Downstream products
-
25108-56-9

4-isopropenyldimethylaniline
-
92423-36-4

ethyl 4-(N,N-dimethylamino)cyclohexanecarboxylate
-
113511-05-0

p-EtO2C-C6H4NMe3(+)*I(-)
-
19620-56-5

p,p'-Bis-(n-propyl)-p'-dimethylamino-triphenylcarbinol
Relevant Products
-
Flumazenil
CAS:78755-81-4
-
Icaritin
CAS:118525-40-9