Product Details;
CasNo: 127-06-0
Molecular Formula: C3H7NO
Appearance: white crystals
127-06-0 Properties
- Molecular Formula:C3H7NO
- Molecular Weight:73.0947
- Appearance/Colour:white crystals
- Vapor Pressure:4.65mmHg at 25°C
- Melting Point:60-63 °C(lit.)
- Refractive Index:1.41
- Boiling Point:135 °C at 760 mmHg
- PKA:12.2(at 25℃)
- Flash Point:45.2 °C
- PSA:32.59000
- Density:0.91 g/cm3
- LogP:0.85640
127-06-0 Usage
Physical and Chemical Properties
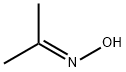
Acetone oxime (Abbreviation DMKO for short), also known as dimethyl ketone oxime, is a white flaky crystal at room temperature, relative density: 0.9113, melting point: 60 ℃, flash point: 47.2 ℃, boiling point: 134.8 ℃, toxicity LD50: 5500mg/kg. It is soluble in water and alcohol, ether and other solvents, saturated aqueous solubility is 25% (mass percentage), its aqueous solution is neutral, it hydrolyzes easily in dilute acid, can make potassium permanganate fading at room temperature. Mainly used as chemical oxygen scavenger for industrial boiler feed water, compared with the traditional boiler chemical oxygen scavenger, it has characteristics of less dosage, high oxygen removal efficiency, non-toxic, pollution-free. It is the best drug for the outage protection and passivation treatment of subcritical boiler, also is the ideal products of substituted hydrazine and other traditional chemical oxygen scavengers in medium and high pressure boiler feed water. Figure 1 the molecular structure of Acetone oxime.
Chemical Properties
It is white needle-like crystal. Melting point is 61 ℃, boiling point is 136 ℃, 134.8 ℃ (97.1kPa), 61 ℃ (2.67kPa), the relative density is 0.9113 (62/4 ℃), refractive index is 1.4156. Easily soluble in water, ethanol, ether and acetone, soluble in acid, easily hydrolyzed in dilute acid. It volatilize in the air quickly.
Uses
1. Acetone oxime is used in organic synthesis, as an analytical reagent, used for the determination of cobalt. 2. Used as the intermediates of Caffeine, theophylline, SMD 3. Acetone oxime is used as test reagents for Chromium, used for organic synthesis, used as novel oxygen scavenger of boiler water, intermediates of medicine, pesticide. 4. It is used as raw materials of pharmaceutical, pesticide, dyes and organic silane coupling agent, can also be used as analytical reagent to identify nickel, cobalt, etc.
chemical reaction
Acetone oxime has a strong reduction, it is easy to react with oxygen in water to reduce the dissolved oxygen content in water, reaction is as follows: 2C3H7NO + O2 → 2C3H6CO + N2O + H2O and 4 (CH3) 2C = N-OH + O2 → 4 (CH3) 2C = O + 2N2 + H2O Meanwhile, Acetoxime also reacts with the metal for passivation, reaction is as follows: 2C3H7NO + 6Fe2O3 → 2C3H6CO + N2O + 4Fe3O4 + H2O Acetoxime can reduce the content of iron in the feed water, to prevent overheating of the metal pipe and corrosion damage of the boiler due to the formation of iron oxide deposits, while with the cleaning effect for copper corrosion products deposited on pipes, economizer, etc. This is the reason that in the early use of acetone oxime, the content of copper in boiler water will be significantly higher. Decomposition products of Acetoxime are mainly nitrogen and water, a small amount of formic acid, acetic acid, nitrogen oxides and so on. On the premise of ensuring oxygen removal effect, when the residual amount of DMKO in feed water is controlled to be 5~40μg/L, the formic acid, acetic acid, Cl-, SO42 + was not detected in all tested samples of water vapor, at the same time NO2-and NO3-content of same samples were tested, are also not detected. Therefore, there is no any adverse effects for the use of acetone oxime oxygen in vapor system.
Production method
It is obtained by the reaction of acetone with hydroxylamine hydrochloride. The hydroxylamine hydrochloride solution was slowly added dropwise in acetone, the reaction temperature is controlled at 40-50 ℃. The oximation reaction liquild was neutralized by 40% sodium hydroxide up to basic (pH7-8), cooling and filtration, the crude product was filtered off and add the zeolite, atmospheric distillation, cooling to obtain the finished product crystals.
Passivation agent after Boiler pickling
After the boiler pickling, the metal surface has high activity, it is necessary to use passivating agent to generate dense protective film on metal surfaces to prevent secondary corrosion of metal. Multi-hydrazine, sodium nitrite, sodium tripolyphosphate, etc, is conventionally used as passivating agent. Although the hydrazine and sodium pin Asia have a good passivation effect, but the drug itself has significant side effects to operators and users, it is difficult to handle passivation solution, and it pollutes the environment. Although Passivation method of sodium tripolyphosphate has advantages that its process is simple, liquid waste is easy to handle, but easily lead to the boiler water PH value lower after the unit started, bring some difficulties to control and process the water vapor quality. The experiment proved that replacement of the above passivating agents by acetone oxime (dimethyl ketone oxime) can obtain a satisfactory or better result. And it has the advantages of less dosage, emissions non-toxic pollution-free and so on. General passivation parameters: The concentration of Passivating agent: 8000-900mg/l PH value (ammonia tone) of passivation solution: 9.50-11 The temperature of purified fluid (atmospheric pressure cleaning system): 85-90 Purification Time: 14-18h
Thermal Equipment Disable protection agent
Due to this product has a strong reduction, the solution can form a good magnetic film on the steel surface, thereby effectively delay corrosion during downtime of the thermal equipment. The solution containing acetone oxime (dimethyl ketone oxime) can be implemented in wet protection, can obtain significant inhibition effect. concentration of Protection liquild: 350-400mg/l (water preparation) PH:> 10.5 (ammonia adjustment) During protection, should note: 1. Due to sampling and other reasons that result in loss of protective agents, use dosing devices regularly serviced. 2. The samples tested once a week or a half months, if the concentration of protection liquild is stable, the slow decline for the concentration of iron and oxygen is a normal phenomenon, on the contrary should pinpoint the cause.
Hazards & Safety Information
Category: Oxidant Toxicity grading: Moderately toxic Acute toxicity Oral-rat LD50:> 500 mg/kg, intraperitoneal-Mouse LD50: 4000 mg/kg Flammability hazard characteristics: In case of fire, it is combustible. Thermal decomposition releases nitrogen oxide gases. Storage Characteristics: Treasury ventilation, low temperature drying, light loading and unloading, it is stored separately from oxidant and acid. Extinguishing agent: foam, Carbon dioxide, dry powder, sand
Chemical Properties
White crystals
Uses
It is an intermediate used in organic synthesis and in agriculture. It is also an important raw material.
Safety Profile
Moderately toxic by ingestion andintraperitoneal routes. When heated to decomposition itemits toxic fumes of NOx.
Purification Methods
It crystallises from pet ether (b 40-60o) and can be sublimed. [Beilstein 1 H 649, 1 IV 3202.]
InChI:InChI=1/C3H7NO/c1-3(2)4-5/h5H,1-2H3
127-06-0 Relevant articles
-
Stewart
, p. 410 (1905)
-
-
Ogata,Hirano
, (1930)
-
Rates of Formation of Iminium Ions from Acetone and Monoprotonated 2-pyrrolidine
Hine, Jack,Evangelista, Ramon A.
, p. 3890 - 3892 (1980)
The kinetics of the reaction of 2-pyrrolidine (1) with acetone has been studied by experiments in which the reversibly formed iminium ion is captured irreversibly by hydroxylamine.From experiments over the pH range 8.5-10.6 rate constants for iminium ion formation from 1 and 1-H+ were obtained.These rate constants were smaller than the corresponding rate constant for pyrrolidine, but the value for 1-H+ was large enough to show that the intermediate carbinolamine was undergoing internal acid-catalyzed dehydration to give the iminium ion.
Metal-free artificial nucleases based on simple oxime and hydroxylamine scaffolds
Fernandes, Luciano,Fischer, Franciele L.,Ribeiro, Carolina W.,Silveira, Gustavo P.,Sa, Marcus M.,Nome, Faruk,Terenzi, Hernan
, p. 4499 - 4502 (2008)
Hydrolysis of DNA is of increasing importance in biotechnology and medicine. In this Letter, we present the DNA-cleavage potential of metal-free hydroxylamines and oximes as new members of nucleic acid cleavage agents.
Selective synthesis of dimethyl ketone oxime through ammoximation over Ti-MOR catalyst
Ding, Jianghong,Wu, Peng
, p. 86 - 95 (2014)
Titanosilicate with the MOR topology (Ti-MOR), postsynthesized from highly dealuminated mordenite and TiCl4 vapor through a solid–gas reaction, was highly active and selective for the liquid-phase ammoximation of dimethyl ketone (DMK) with ammonia and hydrogen peroxide. The parameters effecting the formation of the ammoximation product of dimethyl ketone oxime were investigated systematically in a batch-type reactor, and the optimized conditions were further applied to continuous ammoximation of DMK in a slurry reactor. Ti-MOR was superior to other titanosilicates in terms of activity and lifetime. TS-1 was not suitable for the ammoximation of DMK, whereas Ti-MWW required a higher catalyst loading to reach a reasonable activity, and they both easily produced a main byproduct of oxidative coupling of dimethyl ketone oxime. The deactivation behavior of Ti-MOR was investigated. Ammonia-induced structural desilication and accompanied Ti sites migration altered a more serious influence on the catalyst duration than coke deposition during continuous ammoximation.
Stereochemical and electronic interaction studies of α-heterosubstituted acetone oximes
Olivato, P. R.,Ribeiro, D. S.,Rittner, R.,Hase, Y.,Pra del, D.,Bombieri, G.
, p. 1479 - 1496 (1995)
The free νC=N bands in the IR spectra of some α-heterosubstituted acetone oximes show the existence of only a monomeric form in chloroform solutions below 1E-2 M, while in carbon tetrachloride self-associated species are also present.The 1H and 13C NMR chemical shift data indicate the predominance of the E over the Z isomer.The ΔνC=N frequency shifts and molecular mechanics strongly suggest that the oximes are in the gauche conformation.X-ray diffraction data have shown that the single dimethylaminoacetone oxime isomer exists in the E configuration and gauche conformation.Non-additivity effects for the α-methylene carbon chemi cal shifts seem to indicate the occurence of a ?C=N/?*C-x interaction besides the ?*C=N/?C-X hyperconjugative interaction.
Influences of fluorine implantation on catalytic performance and porosity of MOR-type titanosilicate
Yang, Yulin,Ding, Jianghong,Wang, Binshen,Wu, Jing,Zhao, Chen,Gao, Guohua,Wu, Peng
, p. 160 - 169 (2014)
Fluorine species were implanted into the framework of Ti-MOR by post-treatment with fluorides in order to modify the microenvironment around Ti active sites and then to improve their catalytic activity in liquid-phase oxidation. The effects of NH4F amount, fluorination temperature, and solvent on the catalytic performance of F-Ti-MOR were investigated in detail. Methanol was found to be superior to water in fluorination. Fluorine implantation increased the electropositivity of Ti active sites through forming SiO3/2F units in the neighborhood, which enhanced the catalytic performance in the ammoximation of cyclohexanone remarkably. F-Ti-MOR prepared under optimized fluorination conditions showed a cyclohexanone conversion of 99% in comparison to only 30% conversion given by primitive Ti-MOR. Meanwhile, the implanted fluorine species captured the organic molecules with a relatively large dimension tightly, creating enormous steric hindrances that prevented other molecules, in particular those with bulky molecular dimensions, from diffusing into channels freely. Thus, F-Ti-MOR showed much lower activity than Ti-MOR in the hydroxylation of aromatics.
Kinetics and mechanism of the copper-catalysed oxygenation of 2-nitropropane
Balogh-Hergovich, Eva,Greczi, Zoltan,Kaizer, Jozsef,Speier, Gabor,Reglier, Marius,Giorgi, Michel,Parkanyi, Laszlo
, p. 1687 - 1696 (2002)
Primary and secondary nitro compounds react with dioxygen in the presence of copper metal and N ligands such as N,N,N′,N′-tetramethylethylenediamine (tmeda), 2,2′-bipyridine (bpy), and 1,10-phenantroline (phen) in various solvents to form aldehydes or ketones. More coordinating solvents as well as donor N ligands accelerate the reaction remarkably. The oxygenolysis of 2-nitropropane (NPH) in the presence of copper and tmeda in DMF results in acetone and acetone oxime. The amount of tmeda influences the chemoselectivity, higher tmeda concentrations preferentially lead to the formation of the oxime. The kinetics of the reaction, measured at 90 °C, resulted in a rate equation of first-order dependence on copper and dioxygen and second-order dependence on 2-nitropropane. The rate constant, activation enthalpy, and entropy at 363.16 K are as follows: kcat = (5.37 ± 0.34) × 10-2 Mol-3 dm9 s-1, Ea = 131 ± 4 kJ mol-1, ΔH? = 127 ± 4 kJ mol-1 and ΔS? = 80 ± 13 J mol-1 K-1. The catalytically active intermediates CuII(NP)2(tmeda) and CuII(NO2)2(tmeda) in the catalytic cycle were isolated and their structures determined by X-ray crystallography. The kinetics of the stoichiometric oxygenation of CuII(NP)2(tmeda) to CuII(NO2)2(tmeda) and acetone resulted in the overall second-order rate equation with a rate constant, activation enthalpy, and entropy at 313.16 K of ks = 0.46 ± 0.02 mol-1 dm3 s-1, Ea = 38 ± 1 kJ mol-1, ΔH? = 35 ± 1 kJ mol-1 and ΔS? = -142 ± 13 J mol-1 K-1, respectively. Wiley-VCH Verlag GmbH, 69451 Weinheim, Germany, 2002.
-
Cocivera,M.,Woo,K.W.
, p. 7366 - 7371 (1976)
-
-
Gowenlock et al.
, p. 3587,3588 (1973)
-
-
Francesconi,Milesi
, p. I, 425 (1902)
-
Cyclization of N-allylthiourea derivatives by the action of α-chloronitrosoalkanes
Tkachenko,Pushin,Sokolov,Fedoseev,Martynov
, p. 347 - 350 (1998)
A convenient method is proposed for obtaining difficultly available derivatives of 2-amino-5-chloromethyl-2-thiazoline by the cyclization of N-allylthioureas under the action of α-chloronitrosoalkanes. It is assumed that the reaction proceeds as a halogenophilic process leading to the intermediate formamidinesulfenyl chloride which is rapidly and selectively cyclized with the formation of 2-amino-2-thiazoline derivatives. 1998 Plenum Publishing Corporation.
Six-at-Once Dedeuteration of Acetone-d6 in the Presence of 3-exo--2-exo-norbornanamine
Hine, Jack,Tsay, Hwai-Min
, p. 3797 - 3802 (1983)
3-exo--2-exo-norbornanamine (2) was prepared and used as a catalyst in aqueous solution at 35 degC for the dedeuteration of acetone-d6.Monoprotonated 2 acts as an effective catalyst by transforming the ketone to an iminium ion and then using its dimethylamino group to dedeuterate the iminium ion by internal basic catalysis.At pH 9.95 the most common result of iminium-ion formation is the exchange of all six deuterium atoms.This requires a mechanism for cis-trans isomerization of the intermediate iminium ion.The gem-diamine mechanism proposed earlier for iminium ions derived from cyclopentanone helps explain why 2 gives six-at-once exchange while some rather similar diamines do not.The kinetics of iminium-ion formation from 2 and acetone were studied by the hydroxylamine-capture technique.The rates of iminium-ion formation thus obtained are reasonably consistent with those obtained in the deuterium exchange experiments.
-
Prati
, p. 310 (1894)
-
-
Fitzpatrick,Gettler
, p. 530,533 (1956)
-
-
Menard,Aston
, p. 1601 (1934)
-
Nickel-Catalyzed NO Group Transfer Coupled with NOxConversion
Padmanaban, Sudakar,Choi, Jonghoon,Vazquez-Lima, Hugo,Ko, Donghwi,Yoo, Dagyum,Gwak, Jinseong,Cho, Kyung-Bin,Lee, Yunho
supporting information, p. 4585 - 4593 (2022/03/02)
Nitrogen oxide (NOx) conversion is an important process for balancing the global nitrogen cycle. Distinct from the biological NOx transformation, we have devised a synthetic approach to this issue by utilizing a bifunctional metal catalyst for producing value-added products from NOx. Here, we present a novel catalysis based on a Ni pincer system, effectively converting Ni-NOx to Ni-NO via deoxygenation with CO(g). This is followed by transfer of the in situ generated nitroso group to organic substrates, which favorably occurs at the flattened Ni(I)-NO site via its nucleophilic reaction. Successful catalytic production of oximes from benzyl halides using NaNO2 is presented with a turnover number of >200 under mild conditions. In a key step of the catalysis, a nickel(I)-?NO species effectively activates alkyl halides, which is carefully evaluated by both experimental and theoretical methods. Our nickel catalyst effectively fulfills a dual purpose, namely, deoxygenating NOx anions and catalyzing C-N coupling.
Rhodium(III)-Catalyzed Direct C-H Arylation of Various Acyclic Enamides with Arylsilanes
Li, Xiaolan,Sun, Kai,Shen, Wenjuan,Zhang, Yong,Lu, Ming-Zhu,Luo, Xuzhong,Luo, Haiqing
supporting information, p. 31 - 36 (2021/01/09)
The stereoselective β-C(sp2)-H arylation of various acyclic enamides with arylsilanes via Rh(III)-catalyzed cross-coupling reaction was illustrated. The methodology was characterized by extraordinary efficacy and stereoselectivity, a wide scope of substrates, good functional group tolerance, and the adoption of environmentally friendly arylsilanes. The utility of this present method was evidenced by the gram-scale synthesis and further elaboration of the product. In addition, Rh(III)-catalyzed C-H activation is considered to be the critical step in the reaction mechanism.
Visible-Light-Mediated Strategies for the Preparation of Oxime Ethers Derived from O-H Insertions of Oximes into Aryldiazoacetates
Duarte, Marcelo,Jurberg, Igor D.,Le?o, Luiz Paulo M. O.,Saito, Felipe A.,Stivanin, Mateus L.
supporting information, p. 17528 - 17532 (2021/12/02)
Two visible-light-mediated O-H insertion protocols involving oximes and aryldiazoacetates leading to different products depending on the solvent employed are reported. In DCM, direct O-H insertion takes place. In THF, there is the additional incorporation of the ring-opened form of this solvent into the structure of the product. These metal-free protocols are mild and tolerant to air and moisture. The preparation of an acaricide has been developed as an example of synthetic application.
Annulation of Oxime-Ether Tethered Donor–Acceptor Cyclopropanes
Irwin, Lauren C.,Allen, Meredith A.,Vriesen, Matthew R.,Kerr, Michael A.
, p. 171 - 175 (2019/12/24)
Novel oxime-ether tethered cyclopropanes, when exposed to Yb(OTf)3 and heat, annulate to generate hydropyrrolo-oxazines products that can be taken to their respective pyrrolidines via hydrogenative N?O bond cleavage. The hydropyrrolo-oxazines are generated in a diastereoselective manner isolating the cis or trans product based on the temperature of the reaction. 20 examples of selective cis and trans hydropyrrolo-oxazines were generated in high yields by temperature control.
127-06-0 Process route
-

- 79-46-9
2-nitropropane

-

- 13880-55-2
N,N,N',N'-tetramethyl-C-phenylmethanediamine

-

- 611-74-5
N,N-dimethylbenzamide

-

- 127-06-0
acetone oxime
| Conditions | Yield |
|---|---|
|
at 120 - 130 ℃;
|
|
|
|
-

- 932-90-1,622-31-1,140461-24-1,140461-25-2,622-32-2
syn-benzaldehyde oxime

-

- 67-64-1
acetone

-

- 100-52-7
benzaldehyde

-

- 127-06-0
acetone oxime
| Conditions | Yield |
|---|---|
|
With perchloric acid; In water; water-d2; at 23 ℃; for 24h; Inert atmosphere;
|
29% |
127-06-0 Upstream products
-
60-29-7

diethyl ether
-
2421-26-3

2-chloro-2-nitrosopropane
-
544-97-8

dimethyl zinc(II)
-
7119-91-7

2-bromo-2-nitroso-propane
127-06-0 Downstream products
-
308293-65-4

acetone-(O-biphenyl-4-carbonyl oxime )
-
73826-09-2

rac-propan-2-one-O-(2-hydroxy-2-phenylethyl)oxime
-
18312-45-3

propan-2-one O-acetyl oxime
-
113038-82-7

N-(E)-cinnamyl-hydroxylamine
Relevant Products
-
Pyridinium,1-hexadecyl-, chloride, hydrate (1:1:1)
CAS:6004-24-6