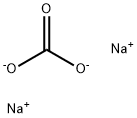
497-19-8
- Product Name:Sodium carbonate
- Molecular Formula:Na2CO3
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 497-19-8
Molecular Formula: Na2CO3
Appearance: white odourless powder
497-19-8 Properties
- Molecular Formula:Na2CO3
- Molecular Weight:105.989
- Appearance/Colour:white odourless powder
- Melting Point:851 °C
- Refractive Index:1.535
- Boiling Point:333.6 °C at 760 mmHg
- PKA:(1) 6.37, (2) 10.25 (carbonic (at 25℃)
- Flash Point:169.8 °C
- PSA:63.19000
- Density:2.54 g/cm3
- LogP:-2.44700
497-19-8 Usage
Description
Sodium carbonate, Na2CO3, is a sodium salt of carbonic acid. The pure product appears as a while, odorless powder with a strong alkaline taste. It has high hygroscopicity. It can be easily dissolved in water to form an aqueous solution with moderate alkalinity. Sodium carbonate has wide applications in various kinds of fields around the world. One of most important application of sodium carbonate is for the manufacturing of glass. Based on statistics information, about half of the total production of sodium carbonate is used for the manufacturing of glass. During the production of glass, sodium carbonate acts as a flux in the melting of silica. In addition, as a strong chemical base, it is used in the manufacturing of pulp and paper, textiles, drinking water, soaps and detergents and as a drain cleaner. In addition, it can also be used for tissue digestion, dissolving amphoteric metals and compounds, food preparation as well as acting as a cleaning agent. There are generally two ways for the production of sodium carbonate. One is through the reactions between sodium chloride and calcium carbonate (via the ammonia soda (Solvay) process). The other is from sodium carbonate and hydrogencarbonate ores (trona and nahcolite).
Physical Properties
Sodium carbonate is an inorganic salt and therefore the vapour pressure can be considered negligible. It has a melting point of 851°C (CRC Handbook, 1986; The Merck Index, 1983), it decomposes when heated at > 400 °C and therefore a boiling point cannot be determined. soluble in water; insoluble in alcohol; dissolves in acids liberating CO2.The octanol water partition coefficient (log Pow) is not relevant for an inorganic substance which dissociates. The average particle size diameter (d50) of light sodium carbonate is in the range of 90 to 150 μm and of dense sodium carbonate is in the range of 250 to 500 μm. The monohydrate consists of colorless and odorless small crystals or cystalline powder; orthorhombic structure; refractive index 1.420; hardness 1.3 Mohs; density 2.25 g/cm3; loses water at 100°C becoming anhydrous; very soluble in water; insoluble in ethanol. The decahydrate consists of transparent crystals; effloresces on exposure to air; density 1.46 g/cm3; decomposes at 34°C; very soluble in water; insoluble in ethanol. An aqueous solution of sodium carbonate are strongly alkaline.
Chemical Properties
Sodium carbonate is a white, crystalline and hygroscopic powder with a purity of > 98 %. There are two forms of sodium carbonate available, light soda and dense soda. Impurities of sodium carbonate may include water (< 1.5 %), sodium chloride (< 0.5 %), sulphate (< 0.1 %), calcium (< 0.1 %), magnesium (< 0.1 %) and iron (< 0.004 %). The purity and the impurity profile depends on the composition of the raw materials, the production process and the intended use of the product. For example the purity of the pharmaceutical grade must be higher than 99.5 % in Europe. Sodium carbonate is a strong alkaline compound with a pH of 11.6 for a 0.1M aqueous solution (The Merck Index, 1983; Johnson and Swanson, 1987). The pKa of CO3 2- is 10.33, which means that at a pH of 10.33 both carbonate and bicarbonate are present in equal amounts.
Uses
Sodium carbonate is a kind of important raw material for chemical industry with wide application. It is the important raw material for making glass, soaps, detergents, textiles, leather, spices, dyes, medicines, etc. It can be used for analysis reagents and also used for the pharmaceutical industry and photoengraving. It is widely used in glass, chemicals, paper making, metallurgy, pharmaceutical, and textile as well as food industries. It is TV dedicated reagent. It can be used for the food industry as the neutralizing agent, leavening agents such as for the manufacture of amino acids, soy sauce and pasta such as bread, bread and so on. It can also be prepared to dubbed alkaline and add into pasta to increase the flexibility and ductility. As the detergent, it can be used for wool rinse. It can also be applied to bath salts and pharmaceutical use and also be used as the alkali agent of tanning. Sodium carbonate is most used in industry with a small part using by the civilian. In the soda ash of industry purpose, it is mainly applied to light industry, building materials and chemical industry, accounting for about 2/3: followed by metallurgy, textiles, petroleum, defense, and pharmaceutical. The glass industry is the largest soda consumer sector with each ton of glass consuming 0.2 ton of soda ash. In the chemical industry, it can be used for manufacturing of sodium silicate, sodium dichromate, sodium nitrate, sodium fluoride, baking soda, borax, and trisodium phosphate. In the metallurgical industry, it is mainly used for fluxing agent, mineral flotation agent, and desulfurization agent for steel and antimony. It can also be used as water softener in printing and dyeing industry. In tanning industry, it can be used for the degreasing of raw hides, neutralizing chrome tanned leather and improving the alkalinity of the chrome liquid. It is also used in the production of synthetic detergent additive sodium tripolyphosphate and other sodium salt. It can be used as a buffer, neutralizing agent and dough conditioner. It can be used in cakes and pastas. Make appropriate use it according to actual requirement of production. It is mainly applied to float glass, funnels, optical glass. It can also be used in other sectors of chemical industry and metallurgy industry. It can reduce the flying the alkali dust through application of heavy soda ash, and thus reducing the material consumption, improving the working conditions as well as improving product quality while reducing its erosion on the refractory material to extend the life of the furnace. It is a kind of basic chemical raw material which is widely used in medicine, paper making, metallurgy, glass, textiles, dyes and other industries and can be used as a leavening agent in food industry. It can be used as analytical reagents, dehydrating agent, and battery additives.
Biological Functions
Sodium carbonate is used as a buffer component in such applications as chromatography, capillary electrophoresis, and enzyme catalysis. Sodium carbonate is widely used in the isolation of cell membranes, membrane proteins, and hydrophobic proteins. A protocol for the isolation of polyamines from cell culture media has been published.
Toxicity
ADI (acceptable daily intake) make no restrictions (FAO/WHO in 1985). LD50 (median lethal dose) is about 6 g/kg (mice-oral). Soda ash dust has irritation effects on the skin, respiratory and eyes. Long-term exposure to soda solution may cause eczema and dermatitis. Its concentrated solutions can cause burns, necrosis, and even corneal opacity. The maximal allowable concentration of soda ash dust in the air is 2 mg/m3. The operators should wear overalls, door cover, gloves, boots and other protective clothing to protect the respiratory system and skin.
Production method
Sodium carbonate at present is mostly mined from its natural deposits. It also is manufactured syntheticallly by Solvay (or ammonia-soda) process. The natural production of sodium carbonate currently has supassed its synthetic production. The Solvay process involves a series of partial reactions. The first step is calcination of calcium carbonate to form lime and CO2. Lime is converted to calcium hydroxide. The most crucial step of the process involves reacting brine solution with carbon dioxide and ammonia to produce sodium bicarbonate and ammonium chloride. Sodium bicarbonate converts to sodium carbonate. The calcium hydroxide and ammonium chloride react to form calcium chloride as the by-product. The partial reactions are shown below: CaCO3 → CaO + CO2 CaO + H2O → Ca(OH)2 2NaCl + 2CO2 + 2NH3 + 2H2O → 2NaHCO3 + 2NH4Cl 2NaHCO3 → Na2CO3 + H2O + CO2 Ca(OH)2 + 2NH4Cl → CaCl2 + 2NH3 + 2H2O The overall reaction: CaCO3 + 2NaCl → Na2CO3 + CaCl2 Sodium carbonate was made historically by the Leblanc process. The first commercial production was carried out by the Leblanc process. In this process, sodium chloride was treated with sulfuric acid to produce sodium sulfate and hydrochloric acid. Heating the sodium sulfate with coal and limestone produced a “black ash” that contained sodium carbonate, calcium sulfide, unreacted coal, and calcium carbonate. Sodium carbonate was separated from the black ash by leaching with water. The overall reaction is as follows: Na2SO4 + 2C + CaCO3 → Na2CO3 + CaS + 2CO2
References
https://en.wikipedia.org/wiki/Sodium_hydroxide#Uses http://www.essentialchemicalindustry.org/chemicals/sodium-carbonate.html
Description
Sodium carbonate is known as soda ash or washing soda and is a heavily used inorganic compound. Approximately 45 million tons of soda ash are produced globally both naturally and synthetically. Soda ash is obtained naturally primarily from the mineral trona, but it can also be obtained from nahcolite (NaHCO3) and salt brine deposits. Trona is a freshwater sodium carbonate-bicarbonate evaporite, with the formula Na3CO3HCO3 .2H2O. The largest known deposit of trona is located in the Green River area of Wyoming, and other large deposits are found in Egypt’s Nile Valley and California’s Searles basin around the city of Trona. Soda ash is produced from mined trona by crushing and screening the ore and then heating it. Th is produces a soda ash mixed with impurities. Pure soda ash is obtained by dissolving the product and precipitating impurities combined with filtering processes.
Chemical Properties
Sodium carbonate, Na2C03, also known as soda or soda ash,is the most important of the industrial alkalis. It is a white or grayish-white, lumpy, water-soluble powder that loses its water of crystallization when heated. It decomposes at a temperature of about 852°C (1560°F). It exists in solution only. It is prepared by the combination of carbon dioxide and water.
Occurrence
Ash is a tree found in regions of North America
History
Sodium carbonate, Na2CO3, has been used historically for making glass, soap, and gunpowder. Along with potassium carbonate, known as potash, sodium carbonate was the basis of the alkali industry, which was one of the first major chemical industries. Throughout history, alkalis were obtained from natural sources. Soda ash was also produced by burning wood and leaching the ashes with water to obtain a solution that yielded soda ash when the water was boiled off. The name soda ash originates from the barilla plant, which was used to produce soda ash. The scientific name of this plant is Salsola soda, but it goes by the common names of sodawort or glasswort because the soda produced from it was used in making glass. Barilla is a common plant found in saline waters along the Mediterranean Sea in Spain and Italy. Barilla was dried and burned to produce soda ash. The depletion of European forests and international disputes made the availability of alkali salts increasingly uncertain during the latter part of the 18th century. LeBlanc proposed a procedure in 1783, and a plant based on LeBlanc’s method was opened in 1791. Unfortunately, LeBlanc’s association with French Royalty led to the confi scation of the plant at the time of the French Revolution. Furthermore, confl icting claims for LeBlanc’s method were made by several other chemists and he never received the reward.
Uses
Soda ash is used in glass making, in production of sodium chemicals (such as sodium chromates, phosphates, and silicates), in the wood pulp industry, in production of soaps and detergents, in oil refining, in water softening, and in refining of nonferrous metals. In its hydrous crystallized form (Na2C03.10H2O), it is known as sal soda,washing soda,or soda crystals, not to be confused with baking soda,which is sodium hydrogen carbonate or sodium bicarbonate (NaHC03). Its monohydrate form(Na2C03·H20) is the standard compound for scouring solutions. When in solution, sodium carbonate creates less alkalinity than the hydroxides. A 0.1% solution creates a pH of 11;a fully saturated solution is 35%, which has a pH of 12.5. The safety requirements for sodium carbonate, because of its lower alkalinity, can be considered less demanding than those for the related bicarbonates.
Uses
Sodium Carbonate is an alkali that exists as crystals or crystalline powder and is readily soluble in water. it has numerous functions: an antioxidant, a curing and pickling agent, a flavoring agent, a processing aid, a sequestrant, and an agent for ph control. it is used in instant soups to neutralize acidity. it is used in alginate water des- sert gels to sequester the calcium, allowing the alginate to solubilize. it is also used in puddings, sauces, and baked goods.
Uses
Sodium carbonate is also known as washing soda or carbonate of soda, sodium carbonate is a white crystal or powder made by converting salt into sodium sulfate, which was followed by roasting with limestone and coal. It is soluble in water and glycerin but not alcohol. Sodium carbonate was used as a pH modifier in toning baths and as the primary alkali in developers used for gelatin emulsions.
Definition
A dibasic acid formed in small amounts in solution when carbon dioxide dissolves in water: CO2 + H2O?H2CO2 It forms two series of salts: hydrogencarbonates (HCO3–) and carbonates (CO32-). The pure acid cannot be isolated.
Definition
sodium carbonate: Anhydrous sodium carbonate (soda ash, sal soda) is a white powder, which cakes and aggregates on exposure to air due to the formation of hydrates. The monohydrate, Na2CO3·H2O, is a white crystalline material, which is soluble in water and insoluble in alcohol; r.d. 2.532; loses water at 109°C; m.p. 851°C.The decahydrate, Na2CO3·10H2O (washing soda), is a translucent ef?orescent crystalline solid; r.d. 1.44; loses water at 32–34°C to give the monohydrate; m.p. 851°C.Sodium carbonate may be manufactured by the Solvay process or by suitable crystallization procedures from any one of a number of natural deposits, such as:trona (Na2CO3·NaHCO3·2H2O),natron (Na2CO3·10H2O),ranksite (2Na2CO3·9Na2SO4·KCl),pirsonnite (Na2CO3·CaCO3·2H2O),gaylussite (Na2CO3·CaCO3·5H2O).The method of extraction is very sensitive to the relative energy costs and transport costs in the region involved. Sodium carbonate is used in photography, in cleaning, in pH control of water, in textile treatment, glasses and glazes, and as a food additive and volumetric reagent.
Production Methods
Sodium carbonate is produced on all continents of the world from its minerals. It is present in large deposits in Africa and the United States as either carbonate or trona, a mixed ore of equal molar amounts of carbonate and bicarbonate. However, about 70% of the world production of sodium carbonate is manufactured by the Solvay (ammonia soda) process, whereby ammonia is added to a solution of sodium chloride. Carbon dioxide is then bubbled through to precipitate the bicarbonate (NaHCO3) that is decomposed by heat-producing sodium carbonate. In the United States. all production is based on the minerals that contain sodium carbonate. Different qualities of sodium carbonate are produced: technical, food, and pharmaceutical grades.
General Description
Sodium carbonate is a water soluble inorganic salt commonly used as a weak base. Its aqueous solution has the ability to uptake carbon dioxide. It can also catalyze the conversion of sewage sludge to liquid fuels.
Flammability and Explosibility
Nonflammable
Biochem/physiol Actions
Buffer component, may be used for the removal of peripheral membrane proteins.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by inhalation and subcutaneous routes. Mlldly toxic by ingestion. Experimental reproductive effects. A skin and eye irritant. It migrates to food from packagmg materials. Can react violently with Al, P2O5, H2SO4, F2, Li, 2,4,6-trinitrotoluene. When heated to decomposition it emits toxic fumes of Na2O
Purification Methods
It crystallises from water as the decahydrate which is redissolved in water to give a near-saturated solution. By bubbling CO2, NaHCO3 is precipitated. It is filtered off, washed and ignited for 2hours at 280o [MacLaren & Swinehart J Am Chem Soc 73 1822 1951]. Before being used as a volumetric standard, analytical grade material should be dried by heating at 260-270o for 0.5hour and allowed to cool in a desiccator. It has a transition point at 450o, and its solubility in water is 21.58% at 20o (decahydrate in solid phase), 49.25% at 35o (heptahydrate in solid phase) and 44.88% at 75o(monohydrate in solid phase) [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 987-988 1963]. After three recrystallisations, technical grade Na2CO3 had Cr, Mg, K, P, Al, W, Sc and Ti at 32, 9.4, 6.6, 3.6, 2.4, 0.6, 0.2 and 0.2 ppm respectively; another technical source had Cr, Mg, Mo, P, Si, Sn and Ti at 2.6, 0.4, 4.2, 13.4, 32, 0.6, 0.8 ppm respectively.
InChI:InChI=1/CH2O3.2Na/c2-1(3)4;;/h(H2,2,3,4);;/q;2*+1/p-2
497-19-8 Relevant articles
Dehydration of sodium carbonate monohydrate with indirect microwave heating
Seyrankaya, Abdullah,Ozalp, Bari?
, p. 31 - 36 (2006)
In this study, dehydration of sodium carbonate monohydrate (Na2CO3·H2O) (SCM) in microwave (MW) field with silicon carbide (SiC) as an indirect heating medium was investigated. SCM samples containing up to 3% free moisture were placed in the microwave oven. The heating experiments showed that SCM is a poor microwave energy absorber for up to 6 min of irradiation at an 800 W of microwave power. The heat for SCM calcination is provided by SiC which absorbs microwave. The monohydrate is then converted to anhydrous sodium carbonate on the SiC plate by calcining, i.e. by removing the crystal water through heating of the monohydrate temperatures of over 120 °C. The calcination results in a solid phase recrystallization of the monohydrate into anhydrate. In the microwave irradiation process, dehydration of SCM in terms of indirect heating can be accelerated by increasing the microwave field power.
Thermal decomposition of NaHCO3 powders and single crystals. A study by DSC and optical microscopy
Guarini, G. G. T.,Dei, L.,Sarti, G.
, p. 31 - 44 (1995)
The thermal decomposition of four commercial powders and of differently stored single crystals of sodium hydrogen carbonate is studied by power compensation DSC and by optical and FT-IR microscopy. Independently of manufacturer, specified purity and price, the thermal curves of all the commercial powders show a more or less pronounced low temperature peak preceding the one due to the main decomposition. Such small peak is not observed when samples of laboratory recrystallized material are used. However the thermal behaviour of the latter preparation differs remarkably depending on storage conditions: the material kept in closed glass containers decomposes at temperatures higher than those of the material stored in a desiccator in the presence of concentrated H2SO4. The observation by optical microscopy of the behaviour of the surfaces of single crystals coming from different storage conditions when the temperature is raised in a Kofler heater helps the interpretation of the data collected. The mechanism of the decomposition is discussed and the relevant kinetic parameters reported.
Specificity of decomposition of solids in non-isothermal conditions
Vlase,Vlase,Doca,Doca
, p. 597 - 604 (2003)
The thermal stability of the food additives Na metabisulphite, Na and K acetates, glutamic and citric acids, respective of the pharmaceuticals nifedipine and acetyl salicylic acid was studied by means of the non-isothermal kinetic (Friedman differential m
A method of assessing solid state reactivity illustrated by thermal decomposition experiments on sodium bicarbonate
Heda, Pavan K.,Dollimore, David,Alexander, Kenneth S.,Chen, Dun,Law, Emmeline,Bicknell, Paul
, p. 255 - 272 (1995)
The thermal decomposition of sodium bicarbonate (NaHCO3) was studied under different atmospheres (dry nitrogen, air, and carbon dioxide), with various heating rates in order to characterize the substance. Various non-isothermal methods of kinetic analysis were employed in estimating the Arrhenius kinetic parameters, the activation energy and the frequency factor. All show that the most probable reaction mechanism under dry nitrogen and air is the first-order deceleratory mechanism, whereas under carbon dioxide it is the Avrami-Erofeev equation, with n = 1.5. Thermogravimetric and derivative thermogravimetric analysis (TGA and DTG) were employed for comparing the solid state reactivity of different samples of sodium bicarbonate. The reaction parameters, the extent of the reaction (α) and the reaction temperature were used in comparing the reactivities of various samples of sodium bicarbonate differing in particle sizeand surface area produced by grinding the substance in a ball mill. A m ethod was utilized, termed here the α(sample)-α(reference) (α(s)-α(r)) method, by which the solid state reactivity of these samples could be compared with that of a reference. The terms α(s), α(r) refer to the extent of reaction (here the extent of decomposition) at the same temperature for the sample (s) and reference (r).
Interaction of graphite with hydroxide-salt melts
Zarubitskii,Dmitruk,Zakharchenko
, p. 525 - 528 (2006)
The mechanism and kinetics of graphite dissolution in melts based on sodium hydroxide were studied. The effect of various salt additives on the intensity of the occurring reactions is considered. A method recommended for removal of graphite in the form of remainders of molds and mold cores from titanium casts is described. Pleiades Publishing, Inc., 2006.
Microwave-assisted synthesis, crystal structures and thermal behaviour of Na5Y(CO3)4 and Na5Yb(CO3)4·2H2O
Awaleh,Ben Ali,Maisonneuve,Leblanc, Marc
, p. 114 - 120 (2003)
The microwave-assisted synthesis, crystal structure and thermal behavior of two carbonates were discussed. The study was performed using single crystal x-ray diffraction technique. It was found that in both structures Na(1)+ and Yb3+
-
Desjobert, Andre,Petek, Fahrettin
, p. 19 - 23 (1956)
Experiments have shown that it is not advisable to use the crude product of the calcination of sodium bicarbonate as a standardization substance in acidimetry.
Kinetic studies on the thermal decomposition of aluminium doped sodium oxalate under isothermal conditions
Jose John,Muraleedharan,Kannan,Abdul Mujeeb,Ganga Devi
, p. 64 - 70 (2012)
The kinetics of thermal decomposition of sodium oxalate (Na 2C2O4) has been studied as a function of concentration of dopant, aluminium, at five different temperatures in the range 783-803 K under isothermal conditions by thermogravimetry (TG). The TG data were subjected to both model fitting and model free kinetic methods of analysis. The model fitting analysis of the TG data shows that no single kinetic model describes the whole α versus t curve with a single rate constant throughout the decomposition reaction. Separate kinetic analysis shows that Prout-Tompkins model best describes the acceleratory stage of the decomposition while the decay region is best fitted with the contracting cylinder model. Activation energy values were evaluated by model fitting and model free kinetic methods for both stages of decomposition. As proposed earlier the results favours a diffusion controlled mechanism for the isothermal decomposition of sodium oxalate.
Quantitative kinetic and structural analysis of geopolymers. Part 1. the activation of metakaolin with sodium hydroxide
Zhang, Zuhua,Wang, Hao,Provis, John L.,Bullen, Frank,Reid, Andrew,Zhu, Yingcan
, p. 23 - 33 (2012)
Isothermal conduction calorimetry (ICC) is used here to measure the kinetics of geopolymerisation of metakaolin by reaction with NaOH solution under a variety of conditions. Three exothermic peaks are observed in the calorimetric curve, and are assigned to the dissolution of metakaolin, the formation of geopolymer with disordered or locally ordered structure, and finally the reorganization and partial crystallization of this inorganic polymer gels. For the purpose of further quantifying the ICC data, the geopolymeric reaction products are assumed to have an analcime-like local structure, and their standard formation enthalpies are estimated from the available data for this structure. This assumption enables ICC to be used for the first time in a quantitative manner to determine the real reaction kinetics of geopolymerization. Increasing the NaOH concentration up to a molar overall Na/Al ratio of 1.1 is seen to enhance the reaction extent observed at 3 days, up to a maximum of around 40% in the high liquid/solid ratio systems studied here, and accelerates the crystallization process. However, further addition of NaOH does not give any additional reaction within this period, or any further acceleration. Raising the reaction temperature from 25 °C to 40°C increases the initial reaction rate but has little effect on the final reaction extent, particularly when Na/Al > 1.
Thermal Decomposition of Solid Sodium Bicarbonate
Ball, Matthew C.,Snelling, Christine M.,Strachan, Alec N.,Strachan, Rebecca M.
, p. 3709 - 3716 (1986)
The thermal decomposition of solid sodium bicarbonate has been studied in the temperature range 360-500 K over a range of partial pressures of carbon dioxide.The effect of water vapour has also been studied.Above 440 K the reaction follows contracting-cube kinetics with an activation energy of 32 kJ mol-1 and a frequency factor of 101 s-1.In this temperature range the presence of water or carbon dioxide has little effect on the kinetics.Below 390 K the reaction follows first-order kinetics.In nitrogen, the activation energy is ca. 64 kJ mol-1, the frequency factor is 105 s-1 and water vapour has little effect.High partial pressures of carbon dioxide increase the activation energy to ca. 130 kJ mol-1 and the frequency factor to 1013.5 s-1.The results of microscopic examination generally confirm the kinetics but show that at low temperatures in nitrogen and carbon dioxide the process are different in detail.
-
Tananaeff, N. A.,Lasarkevitsch, N. A.
, p. 117 (1930)
-
Synthesis, spectroscopy, single crystal XRD and biological studies of multinuclear organotin dicarboxylates
Hussain, Shabbir,Ali, Saqib,Shahzadi, Saira,Tahir, Muhammad Nawaz,Shahid, Muhammad,Munawar, Khurram Shahzad,Abbas, Syed Mustansar
, p. 64 - 72 (2016)
Multinuclear organotin(IV) dicarboxylates of the general formula (Me3Sn)2L·H2O (1), (Ph3Sn)2L (2) and Me2SnL[Sn(Cl)2Me2]2 (3) were synthesized by refluxing disodium iminodiacetate hydrate (Na2L·H2O) with Me3SnCl/Ph3SnCl/Me2SnCl2 in methanol. The elemental analysis (C, H and N) data agreed well with the chemical compositions of the products. IR spectroscopy demonstrated a bridging coordination mode of the carboxylate group. 1H NMR spectroscopy suggested a penta-coordinated environment around the tin(IV) center in complexes 1 and 3. The title complex 3 represents one of the very few examples of organotin(IV) carboxylates showing simultaneously coordination with dimethyltin(IV) as well as dichlorodimethyltin(IV) moieties, by substitution and addition reactions, respectively. The 13C NMR spectroscopy demonstrated the carboxylate-metal linkages. EIMS and ESI spectra verified the molecular skeletons of the products 1-3. Thermogravimetric analysis revealed the bimetallic nature of 2. A single crystal XRD study of 3 has shown a predominantly square pyramidal geometry with some trigonal bipyramidal characteristics around each metal center. The novel products exhibited antibacterial/antifungal potential and their minimal inhibitory concentrations (MIC) were also evaluated. In vitro hemolytic studies on human red blood cells indicated a slightly toxic nature of the synthesized complexes.
Solid state reaction between dichromates and oxalates
Suba,Udupa
, p. 1197 - 1203 (1989)
The thermal investigation of the reaction taking place between dichromates and oxalates in the solid state has been done taking two systems of potassium dichromate-potassium oxalate and sodium dichromate-sodium by oxalate. The techniques employed include thermogravimetry, differential thermal analysis, infrared spectroscopy and X-ray diffraction studies. The results indicate a stoichiometric reaction of dichromate and oxalate in 1:1 ratio to give the corresponding chromate as the sole product.
Overcoming Crystallinity Limitations of Aluminium Metal-Organic Frameworks by Oxalic Acid Modulated Synthesis
Canossa, Stefano,Gonzalez-Nelson, Adrian,Shupletsov, Leonid,Van der Veen, Monique A.,del Carmen Martin, Maria
, (2020)
A modulated synthesis approach based on the chelating properties of oxalic acid (H2C2O4) is presented as a robust and versatile method to achieve highly crystalline Al-based metal-organic frameworks. A comparative study on this method and the already established modulation by hydrofluoric acid was conducted using MIL-53 as test system. The superior performance of oxalic acid modulation in terms of crystallinity and absence of undesired impurities is explained by assessing the coordination modes of the two modulators and the structural features of the product. The validity of our approach was confirmed for a diverse set of Al-MOFs, namely X-MIL-53 (X=OH, CH3O, Br, NO2), CAU-10, MIL-69, and Al(OH)ndc (ndc=1,4-naphtalenedicarboxylate), highlighting the potential benefits of extending the use of this modulator to other coordination materials.
PbTe nanostructures: Microwave-assisted synthesis by using lead Schiff-base precursor, characterization and formation mechanism
Ahmadian-Fard-Fini, Shahla,Salavati-Niasari, Masoud,Monfared, Azam,Mohandes, Fatemeh
, p. 778 - 788 (2013)
Pure cubic phase lead telluride (PbTe) nanostructures have been produced by using a Schiff-base complex as a precursor in the presence of microwave irradiation. The Schiff base used as ligand was derived from salicylaldehyde and ethylenediamine. The Schiff-base complex was marked as [Pb(salen)]. In addition, the effect of the irradiation time and the type of reducing agent on the morphology and purity of the final products was investigated. The as-synthesized PbTe nanostructures were characterized extensively by techniques like X-ray powder diffraction (XRD), transmission electron microscopy (TEM), and scanning electron microscopy (SEM). The microwave formation mechanism of the PbTe nanostructures was studied by XRD patterns of the products. Although it was found that both ionic and atomic mechanisms could take place for the preparation of PbTe, the main steps were according to the atomic reaction process, which could occur between elemental Pb and Te.
Thermodynamic relations and equilibria in (Na2CO3 + NaHCO3 + H2O): standard Gibbs energies of formation and other properties of sodium hydrogen carbonate, sodium carbonate heptahydrate, sodium carbonate decahydrate, trona: (Na2CO3*NaHCO3*2H2O), and Wegscheider's salt: (Na2CO3*3NaHCO3)
Vanderzee, Cecil E.
, p. 219 - 238 (1982)
Literature results for numerous heterogeneous equilibria in the three-component system (Na2CO3+NaHCO3+H2O) or (Na2CO3+CO2+H2O) were critically evaluated.Careful attention was given to evaluation of activity coefficients and pressure effects.Temperature effects were treated using enthalpies of formation determined previously for the reacting species.Standard Gibbs energies of formation at 298.15 K were evaluated: -(2714.94 +/- 0.30) kJ/mol for Na2CO3*7H2O(s); -(3428.61 +/- 0.40) kJ/mol for Na2CO3*10H2O(s); -(851.30 +/- 0.19) kJ/mol for NaHCO3(s); -(2381.33 +/- 0.55) kJ/mol for trona: Na2CO3*NaHCO3*2H2O(s); -(3604.72 +/- 0.80) kJ/mol for Wegscheider's salt: Na2CO3*3NaHCO3(s).Corresponding standard entropies at 298.15 K are: (422.36 +/- 1.50), (562.74 +/- 1.15), (102.36 +/- 0.80), (303.13 +/- 1.70), and (433.33 +/- 2.50) J/K*mol, respectively, in the same order.The upper temperature limit for existence of trona was evaluated as 395 K.NaHCO3(s) heated in a small closed container forms a saturated solution of NaHCO3(s) and Na2CO3*3NaHCO3(s) at 402 K and above.Likewise, Na2CO3*3NaHCO3(s) heated in a small closed container forms a saturated solution of Na2CO3(s) and Na2CO3*3NaHCO3(s) at 460 K and above.The results permit interpretation of equilibria in the system up to at least 600 K.
Synthesis and characterisation of Na5[CoO2]CO3
Sofin, Mikhail,Peters, Eva M.,Jansen, Martin
, p. 1461 - 1463 (2002)
Na5[CoO2]CO3 was prepared via the azide/nitrate route. Stoichiometric mixtures of the precursors (Co3O4, NaN3, NaNO3 and Na2CO3) were heated in a special regime up to 500°C and annealed at this temperature for 50 h in silver crucibles. Single crystals have been grown by subsequent annealing of the powder at 500°C for 2000 h in silver crucibles, which were sealed in glass ampoules under dry Ar. According to the X-ray analysis of the crystal structure (P4/mmm, Z = 1, a = 4.6467(4), c = 8.2577(6) A?). Na5[CoO2]CO3 is isostructural with Na5[NiO2]CO3 and contains Co1+, which is coordinated by two oxygen atoms forming a dumb-bell. Na5CoCO5 decomposes at 600°C to Na3CoO2 and Na2CO3.
Effect of semiconducting metal oxide additives on the kinetics of thermal decomposition of sodium oxalate under isothermal conditions
Jose John,Muraleedharan,Kannan,Ganga Devi
, p. 71 - 76 (2012)
The effect of semiconducting metal oxide (CuO and TiO2) additives on the kinetics of thermal decomposition of sodium oxalate (Na 2C2O4) to sodium carbonate has been studied at five different temperatures in the
Thermal decomposition of copper(II) and zinc carbonate hydroxides by means of TG-MS: Quantitative analyses of evolved gases
Koga,Tanaka
, p. 725 - 729 (2005)
For the quantitative analyses of evolved CO2and H2O during the thermal decomposition of solids, calibration curves, i.e. the amounts of evolved gases vs. the corresponding peak areas of mass chromatograms measured by TG-MS, were plot
The hidden equilibrium in aqueous sodium carbonate solutions - Evidence for the formation of the dicarbonate anion
Zeller, Klaus-Peter,Schuler, Paul,Haiss, Peter
, p. 168 - 172 (2005)
Crossover 13C NMR experiments between [13C]carbonate and [18O]carbonate in aqueous solution confirm the combined action of two oxygen-exchange modes. The isotopomeric carbon dioxides formed in the hydrolysis equilibrium of
Detection and identification of corrosion products of sodium aluminoborosilicate glasses by 23Na MQMAS and 1H→23Na CPMAS NMR
Egan,Mueller
, p. 9580 - 9586 (2000)
23Na multiple-quantum (MQ) MAS NMR is applicable for monitoring the chemical and structural changes resulting from atmospheric exposure of a series of alkali aluminoborosilicate glasses with compositions RNa2O: 1B2O3:1SiO2:0.25Al2O3 (where R = 0.5-2.5). Glasses with high alkali concentrations possess greater numbers of nonbridging oxygens within the bulk structure and presumably at the initial surface of a fresh sample, and for three samples with R≥1.5 sharp resonances are revealed in the isotropic dimension of an MQMAS NMR experiment conducted after prolonged atmospheric exposure. The MQMAS NMR experiments, combined with 1H→23Na cross-polarization magic-angle spinning (CPMAS) NMR measurements, indicate that these resonances arise from sodium cations no longer participating in the glass network. Two new phases are formed as corrosion products and have been identified as an anhydrous Na2CO3 phase and a NaBO2·1H2O phase through comparison with 23Na MQMAS and 1H→23Na CPMAS NMR spectra of crystalline samples. Due to an inherent difficulty with direct quantification of populations based on MQMAS spectra, a simplified approach for quantification of the amount of the new carbonate phase is presented. Values are then calculated for relative amounts of corrosion product formation for different exposure times and bulk glass compositions.
Thermal Decomposition of Solid Sodium Sesquicarbonate, Na2CO3*NaHCO3*2H2O
Ball, Matthew C.,Snelling, Christine M.,Strachan, Alec N.,Strachan, Rebecca M.
, p. 631 - 636 (1992)
The thermal decomposition of solid sodium sesquicarbonate has been studied at temperatures between 350 and 487 K in nitrogen and carbon dioxide atmospheres.In nitrogen, a single-stage decomposition to sodium carbonate occurs, following Avrami-Erofeyev kinetics (n = 2), with an inflexion at ca. 390 K.The activation energies are 24 kJ mol-1 for the high-temperature region and 58 kJ mol-1 for the low-temperature region.In carbon dioxide above 435 K, the single-stage reaction follows contracting disc kinetics with an activation energy of 29 kJ mol-1.In carbon dioxide at low temperatures, wegscheiderite (Na2CO3*3NaHCO3) and sodium carbonate monohydrate (Na2CO3*H2O) are formed, and this reaction follows first-order kinetics, withb an activation energy of 67 kJ mol-1.Microscopic evidence is also presented.Relationships between the decomposition of sesquicarbonate and other related compounds are discussed.
Study on the thermodynamic properties and dehydration reaction kinetics of some salt-hydrates
Zhang,Wang,Dai
, p. 109 - 115 (1995)
Correlations were determined between heat capacity and temperature and phase change enthalpy of Ba(OH)2·8H2O. The phase diagram and DSC curve of the binary system Na2CO3·10H2O-Na2HPO4
Thermal Decomposition of Sodium Carbonate Perhydrate in the Presence of Liquid Water
Galvey, Andrew Knox,Hood, William John
, p. 2815 - 2828 (1982)
A kinetic study has been made of the decomposition of sodium carbonate perhydrate, Na2CO3.1 1/2 H2O2, in the presence of small quantities of added water at 323-343 K.Reactions were deceleratory throughout and rates in the later stages were further reduced when the quantity of water available was insufficient to permit complete initial dissolution of the reactant solid.Rate coefficients measured for these reactions were compared with similarly determined data for the probable contributory processes.These were the decompositions, in saturated aqueous Na2CO3, of (i) H2O2 and (ii) Na2CO3.1 1/2 H2O2.From the pattern of behaviour observed it was concluded that the reaction of Na2CO3.1 1/2 H2O2 in water proceeds in two stages: heterogeneous dissolution of the reactant crystallites is followed by the homogenous breakdown of H2O2 in solution.This mechanism is distinct and different from the vacuum decomposition of the solid.It is concluded that the rate of the homogenous breakdown of H2O2 is probably controlled by catalytic processes involving transition-metal ions present in solution as impurities.This conclusion is supported by the observation that the present reaction was inhibited by added sodium silicate.The kinetics and mechanisms of these reactions are discussed.The heterogeneous reaction investigated involved both a solid reactant and intermediates dissolved in the added liquid water.This combination of reactants has hitherto been the subject of relatively few detailed kinetic studies.Separate investigations of the individual steps which contribute to the overall change, in this particularly favourable system, has led to the identification of a simple reaction mechanism that is entirely consistent with the observations.The approach demonstrates the value of using complementary rate measurements to characterize the kinetics and mechanism of this decomposition involving both solid and dissolved participants.
Fire retardancy impact of sodium bicarbonate on ligno-cellulosic materials
Bakirtzis,Delichatsios,Liodakis,Ahmed
, p. 11 - 19 (2009)
In this paper, the effect of NaHCO3 as fire retardant additive during pyrolysis and combustion has been investigated. Four different contents (5%, 10%, 15%, and 20% w/w) of NaHCO3 have been tested on Pinus brutia, Laurus nobilis and
Carbon dioxide conversion into the reaction intermediate sodium formate for the synthesis of formic acid
Masood, Muhammad Hanan,Haleem, Noor,Shakeel, Iqra,Jamal, Yousuf
, p. 5165 - 5180 (2020/09/03)
Increased carbon dioxide (CO2) emissions from anthropogenic activities are a contributing factor to the growing global warming worldwide. The economical method to recover and effectively reuse CO2 is through adsorption and absorption. In this study, CO2 is absorbed into the solution of sodium hydroxide having various concentrations (0.01, 0.1, 0.5, 1.0, 3.0 and 5.0?N), and the impact of the solution pH on the various product formation was observed. The resultant products formed at different pH of the absorbing solution are sodium carbonate at pH 10, Trona at pH 9, and sodium hydrogen carbonate at pH 8. The products formed are confirmed through X-ray diffraction analysis. After pH optimization, the sodium hydrogen carbonate formed at pH 8 is converted into sodium formate through hydrogenation in the presence of nickel ferrite catalyst at 80 °C and atmospheric pressure. The sodium formate produced is then used as a precursor to synthesize formic acid upon simple reaction with sulfuric acid. A reaction % age yield of 79 ± 0.2% formic acid is noted. Condensed formic acid vapors are later analyzed, using a high performance?liquid chromatography for the qualitative analysis.
Features of the Thermolysis of Li, Na, and Cd Maleates
Avdin, V. V.,Merzlov, S. V.,Nayfert, S. A.,Polozov, M. A.,Polozova, V. V.,Sakthi Dharan, C. P.,Taskaev, S. V.,Zherebtsov, D. A.
, p. 1311 - 1318 (2020/07/21)
Abstract: Processes of the multi-stage decomposition of maleic acid and Li, Na, and Cd maleates in an inert atmosphere are studied via thermal analysis with synchronous analysis of the composition of the released gases. Reaction mechanisms are proposed according to the data on the mass loss stages determined via thermal analysis, gaseous products, and the final solid decomposition products. It is shown that when heated to 700°C, Li and Na carbonates incorporated into the porous carbon matrix are the final products. Above 350°C, cadmium is reduced from oxide to metal and evaporates to form a porous carbon residue as the only product of thermolysis. All carbon products are X-ray amorphous. Maleic acid decomposes completely into gaseous products in the range of 133–239°C. The maleate ion is more stable in the structure of lithium maleate than in free maleic acid, and Na and Cd cations reduce its stability.
Synthesis, crystal structure and optical properties of a new fluorocarbonate with an interesting sandwich-like structure
Tang, Changcheng,Jiang, Xingxing,Guo, Shu,Xia, Mingjun,Liu, Lijuan,Wang, Xiaoyang,Lin, Zheshuai,Chen, Chuangtian
, p. 6464 - 6469 (2018/05/23)
A new fluorocarbonate, Na3Zn2(CO3)3F, was synthesized using a subcritical hydrothermal method. Na3Zn2(CO3)3F crystallizes in the space group C2/c with a sandwich-like framework in which the stacked [Zn(CO3)]∞ layers are connected with one another by bridging F atoms and [CO3] groups alternately. Interestingly, each Zn atom is surrounded by one F atom and four O atoms, forming a distorted [ZnO4F] trigonal bipyramid, which is observed for the first time in the carbonate system. Na3Zn2(CO3)3F has high transparency in a wide spectral region ranging from UV to mid IR with a short ultraviolet absorption edge (~213 nm). First-principles calculations revealed that Na3Zn2(CO3)3F possesses a large birefringence (Δn = 0.11, λ = 589 nm), which is mainly contributed by the coplanar arrangement of [CO3] groups in the ab plane. Na3Zn2(CO3)3F might find applications as a UV birefringence crystal.
497-19-8 Process route
-

- 141-53-7
sodium formate

-

- 1310-73-2
sodium hydroxide

-

- 1333-74-0
hydrogen

-

- 497-19-8
sodium carbonate
| Conditions | Yield |
|---|---|
|
at 210°C react. slow, at 250°C fast;
|
|
|
above 270°C formed oxalate, which react with NaOH at 260°C slow, at 309°C unmeasurable quickly;
|
|
|
In not given; at melting with an excess of NaOH;;
|
>99 |
|
In not given; heating at 205°C;;
|
>99 |
|
above 270°C formed oxalate, which react with NaOH at 260°C slow, at 309°C unmeasurable quickly;
|
|
|
at 210°C react. slow, at 250°C fast;
|
|
|
|
|
|
|
-

-
disodium tetracarbonylferrate

-

- 124-38-9,18923-20-1
carbon dioxide

-

- 13463-40-6,37220-42-1
iron pentacarbonyl

-

- 497-19-8
sodium carbonate
| Conditions | Yield |
|---|---|
|
In tetrahydrofuran; reductive disproportionation, mechanism discussed;; IR; iron carbonyl not isolated;;
|
94% 82% |
497-19-8 Upstream products
-
7664-41-7

ammonia
-
7732-18-5

water
-
139-33-3

edetate disodium
-
492-62-6

alpha-D-glucopyranose
497-19-8 Downstream products
-
191-28-6

perixanthenoxanthene
-
98-01-1

furfural
-
119-61-9

benzophenone
-
5392-40-5

(E/Z)-3,7-dimethyl-2,6-octadienal
Relevant Products
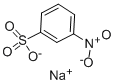
-
Sodium 3-nitrobenzenesulphonateCAS NO.: 127-68-4
CAS:127-68-4
-
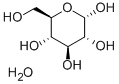
D-Glucose monohydrate
CAS:5996-10-1
-
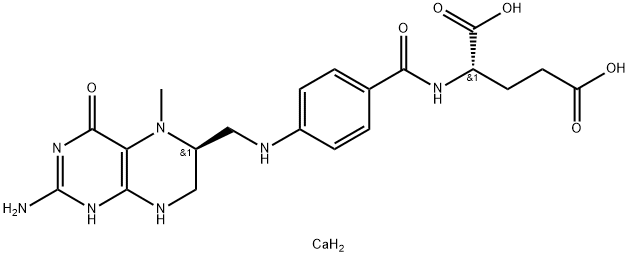
Calcium levomefolate
CAS:151533-22-1