40064-34-4
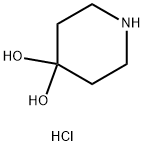
- Product Name:4,4-Piperidinediol hydrochloride
- Molecular Formula:C5H11NO2.HCl
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 40064-34-4
Molecular Formula: C5H11NO2.HCl
Appearance: white crystalline solid
40064-34-4 Properties
- Molecular Formula:C5H11NO2.HCl
- Molecular Weight:153.609
- Appearance/Colour:white crystalline solid
- Vapor Pressure:1.17mmHg at 25°C
- Melting Point:97-99 °C
- Boiling Point:256.8 °C at 760 mmHg
- Flash Point:147.9 °C
- PSA:52.49000
- LogP:0.18150
40064-34-4 Usage
Uses
Piperidine-4,4-diol Hydrochloride is used in preparation of crystal, molecular structure and DFT studies of highly functionalized N-(Pyridinylmethyl)-bis[(E)-arylmethylidene]tetrahydropyridinones.
Description
4-Piperidone (hydrochloride hydrate) (Item No. 21961) is an analytical reference standard categorized as a piperidine. It is a starting material in the synthesis of fentanyl (hydrochloride) (Item Nos. ISO60197 | 14719) with phenethylbromide . The physiological and toxicological properties of this compound are not known. This product is intended for research and forensic applications.
Chemical Properties
White Crystalline Solid
Uses
4-Piperidone monohydrate hydrochloride was used in the generation of compound libraries based on sub-reactions of an Ugi multicomponent reaction (MCR).4-Piperidone (hydrochloride hydrate) is an analytical reference standard categorized as a piperidine. It is a starting material in the synthesis of fentanyl (hydrochloride) with phenethylbromide. The physiological and toxicological properties of this compound are not known. This product is intended for research and forensic applications.
Uses
4-Piperidone hydrochloride monohydrate is used in the generation of compound libraries based on sub-reactions of an Ugi multicomponent reaction (MCR).
Uses
4-Piperidone monohydrate hydrochloride was used in the generation of compound libraries based on sub-reactions of an Ugi multicomponent reaction (MCR).
InChI:InChI=1/C5H9NO/c7-5-1-3-6-4-2-5/h6H,1-4H2/p+1
40064-34-4 Relevant articles
Using the electrostatic field effect to design a new class of inhibitors for cysteine proteases
Conroy, Jeffrey L.,Sanders, Tanya C.,Seto, Christopher T.
, p. 4285 - 4291 (2007/10/03)
A new class of competitive inhibitors for the cysteine protease papain is described. These inhibitors are based upon a 4-heterocyclohexanone ring and are designed to react with the enzyme active site nucleophile to give a reversibly formed hemithioketal. The electrophilicity of the ketone in these inhibitors is enhanced by ring strain and by through-space electrostatic repulsion with the heteroatom at the 1-position of the ring. Equilibrium constants for addition of water and 3-mercaptopropionic acid to several 4- heterocyclohexanones were measured by 1H NMR spectroscopy. These reactions model addition of the active site nucleophile to the corresponding inhibitors. The equilibrium constants give a linear correlation with the field substituent constant F for the functional group at the 1-position of the heterocyclohexanone. These equilibrium constants also correlate well with the inhibition constants for the 4-heterocyclohexanone-based inhibitors, which range from 11 to 120 μM. Thus, the model system can be used to predict the potency of structurally related enzyme inhibitors.
40064-34-4 Process route
-

-
piperidin-4-one; hydrochloride sesquiethylate

-

- 40064-34-4,320589-77-3
4-piperidone monohydrochloride monohydrate
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; water;
|
-

- 41979-39-9
4-piperidone hydrochloride

-

- 40064-34-4
piperidine-4,4-diol hydrochloride
| Conditions | Yield |
|---|---|
|
With water; In water-d2; at 25 ℃; Rate constant;
|
40064-34-4 Upstream products
-
41979-39-9

4-piperidone hydrochloride
40064-34-4 Downstream products
-
34376-54-0

[1,4]diazepan-5-one
-
79099-07-3

N-tert-butyloxycarbonylpiperidin-4-one
-
79098-55-8

3-<1-<2-(3,4-dimethoxyphenyl)-2-oxoethyl>-4-piperidylidene>-1,3-dihydro-2H-indol-2-one
-
79098-62-7

3-{1-[2-(3,4-Dimethoxy-phenyl)-2-hydroxy-ethyl]-piperidin-4-ylidene}-1,3-dihydro-indol-2-one
Relevant Products
-
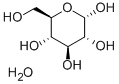
D-Glucose monohydrate
CAS:5996-10-1