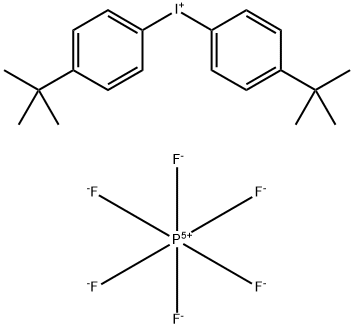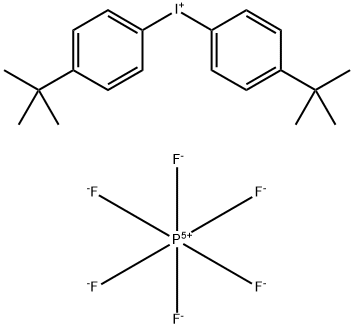
61358-25-6
- Product Name:Bis(4-tert-butylphenyl)iodonium hexafluorophosphate
- Molecular Formula:C20H26F6IP
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 61358-25-6
Molecular Formula: C20H26F6IP
61358-25-6 Properties
- Molecular Formula:C20H26F6IP
- Molecular Weight:538.295
- Melting Point:174 °C
- PSA:13.59000
- LogP:5.79240
61358-25-6 Usage
Uses
Bis(4-(tert-butyl)phenyl)iodonium hexafluorophosphate(V) is an organic catalyst used for the photoinduced thermal polymerization reactions.
InChI:InChI=1/C20H26I.F6P/c1-19(2,3)15-7-11-17(12-8-15)21-18-13-9-16(10-14-18)20(4,5)6;1-7(2,3,4,5)6/h7-14H,1-6H3;/q+1;-1
61358-25-6 Relevant articles
Photoredox-Catalyzed Cascade Reactions Involving Aryl Radical: Total Synthesis of (±)-Norascyronone A and (±)-Eudesmol
Xu, Ze-Jun,Liu, Xu-Yuan,Zhu, Ming-Zhu,Xu, Yu-Liang,Yu, Yue,Xu, Hai-Ruo,Cheng, Ai-Xia,Lou, Hong-Xiang
supporting information, p. 9073 - 9077 (2021/12/06)
Herein, we have developed two types of photoredox-catalyzed cascade reactions using diaryliodonium salts for the concise synthesis of norascyronone A and β-eudesmol. A rationally designed photoredox-catalyzed arylation/cyclization/Friedel-Crafts cascade reaction of enone was exploited to generate the norascyronone polycyclic skeleton. A visible-light-induced radical cyclization/acyloxy-migration reaction was explored to forge the decalin skeleton of eudesmol, and mechanistic studies indicated the reaction was initiated by one-electron oxidation of the enol ester.
61358-25-6 Process route
-

- 253185-03-4,253185-04-5
tert-butylbenzene

-

- 61358-25-6
bis(4-tert-butylphenyl)iodonium hexafluorophosphate
| Conditions | Yield |
|---|---|
|
tert-butylbenzene; With iodine; toluene-4-sulfonic acid; 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 40 ℃; for 0.25h;
With trifluorormethanesulfonic acid; In dichloromethane; at 0 - 20 ℃; for 12h;
With sodium hexaflorophosphate; In dichloromethane; water; at 20 ℃; for 12h;
|
-

- 370-69-4
tris(2,2,2-trifluoroethyl)phosphite

-

- 61358-25-6
bis(4-tert-butylphenyl)iodonium hexafluorophosphate

-

- 1825-33-8
phenylazo-i-butyric acid nitrile

-

- 358-63-4
tris(2,2,2-trifluoroethyl) phosphate

-

- 35779-04-5
1-tert-butyl-4-iodobenzene

-

- 1428647-08-8
bis(2,2,2-trifluoroethyl) tert-butylphenylphosphonate

-

- 172422-37-6
Phenyl-phosphonic acid bis-(2,2,2-trifluoro-ethyl) ester
| Conditions | Yield |
|---|---|
|
With cyclohexane-1,2-epoxide; In neat (no solvent); at 34 ℃; for 4.16667h; UV-irradiation;
|
15 %Spectr. 10 %Spectr. 14 %Spectr. 20 %Spectr. |
|
With cyclohexane-1,2-epoxide; In [D3]acetonitrile; at 34 ℃; for 1h; UV-irradiation;
|
15 %Spectr. 27 %Spectr. 14 %Spectr. 33 %Spectr. |
|
With cyclohexane-1,2-epoxide; In [D3]acetonitrile; at 34 ℃; for 4.5h; UV-irradiation;
|
32 %Spectr. 62 %Spectr. 44 %Spectr. 85 %Spectr. |
61358-25-6 Upstream products
-
253185-03-4

tert-butylbenzene
61358-25-6 Downstream products
-
462-06-6

fluorobenzene
-
591-50-4

iodobenzene
-
22904-43-4

4-tert-Butyl-phenylradikal
-
100-66-3

methoxybenzene
Relevant Products
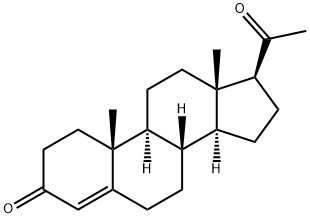
-
Progesterone
CAS:57-83-0