313-06-4
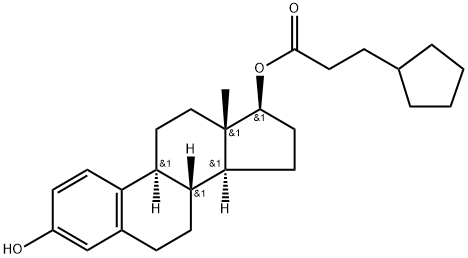
- Product Name:Estra-1,3,5(10)-triene-3,17-diol(17b)-, 17-cyclopentanepropanoate
- Molecular Formula:C26H36O3
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 313-06-4
Molecular Formula: C26H36O3
Appearance: white or off-white crystalline powder
313-06-4 Properties
- Molecular Formula:C26H36O3
- Molecular Weight:396.57
- Appearance/Colour:white or off-white crystalline powder
- Vapor Pressure:5.73E-12mmHg at 25°C
- Melting Point:149-153 °C
- Refractive Index:1.579
- Boiling Point:532.8 °C at 760mmHg
- PKA:10.25±0.60(Predicted)
- Flash Point:207.7 °C
- PSA:46.53000
- Density:1.15 g/cm3
- LogP:6.13050
313-06-4 Usage
Chemical Properties
Estradiol cypionate is a White to Off-White Solid. It is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide (DMF). The solubility of estradiol cypionate in ethanol is approximately 20 mg/ml and approximately 15 mg/ml in DMSO and DMF.
Originator
Depo-Estradiol,Upjohn,US,1952
Uses
Estradiol Cypionate is the cypionate salt form of estradiol, the most potent, naturally produced estrogen. Estradiol Cypionate is present in injectable contraceptive formulations. Used in hormone replacement therapy with potential for improving cognition in post-menopausal women.
Definition
ChEBI: Estradiol 17beta-cyclopentylpropionate is a steroid ester. It inhibits ET-1 synthesis via estrogen receptor.
Manufacturing Process
A solution of 80.0 grams (0.294 mol) of estradiol-17β in 860 ml of pyridine was cooled in an ice-bath and 130.0 grams (0.81 mol) of cyclopentanepropionyl chloride was added dropwise with stirring during a period of about 20 minutes. The ice-bath was removed, stirring was continued for 1 hour and the reaction mixture was allowed to stand at room temperature overnight. The mixture was warmed on a steam bath and stirred for about 45 minutes, cooled and poured slowly onto about 1,000 grams of ice to which had been added 330 ml of concentrated sulfuric acid. The precipitated product was extracted with 400 to 500 mi of ether, and the extract was washed successively with two 100-ml portions of cold 1 N sulfuric acid, two 100-ml portions of saturated sodium carbonate solution and water until the pH was 7 and dried over anhydrous sodium sulfate. After removal of the drying agent, the solution was concentrated to a volume of about 250 ml and an equal volume of methanol was added.After chilling overnight a total of 120.0 grams (78.5%) of estradiol 3,17β- dicyclopentanepropionate was obtained which melted at 87° to 90°C. A sample recrystallized from ether methanol for analysis melted at 90.5° to 91.5°C.To a solution of 2.5 grams (18.1 mmol) of potassium carbonate in 25 ml of water was added 225 ml of methanol followed by 5.0 grams (9.6 mmol) of estradiol 3,17β-dicyclopentanepropionate. The mixture was stirred for 2? hours at 202°C during which time some precipitation occurred. The mixture was poured into 700 ml of water with efficient stirring and the precipitated solid was removed by filtration, washed with water and dried.Recrystallization of the crude product from 80% methanol gave 3.16 grams (83%) of estradiol 17β-cyclopentanepropionate melting at 148° to 151°C. Recrystallization from benzenepetroleum ether raised the MP to 151° to 152°C.
Brand name
depGynogen(Forest); Depo (Pharmacia & Upjohn).
Therapeutic Function
Estrogen
Biological Activity
estradiol cypionate is the 17 β-cyclopentylpropinate ester of estradiol, which inhibits et-1 synthesis via estrogen receptor.
Safety Profile
An experimental teratogen. Other experimental reproductive effects. A steroid. When heated to decomposition it emits acrid smoke and fumes.
Mode of action
Estradiol cypionate diffuses through the cell membrane and binds to and subsequently activates the nuclear estrogen receptor found in the reproductive tract, breast, pituitary, hypothalamus, liver, and bone. The activated complex binds to the estrogen response element on the DNA and activates the transcription of genes involved in the functioning of the female reproductive system and secondary sex characteristics.
InChI:InChI=1/C26H36O3/c1-26-15-14-21-20-10-8-19(27)16-18(20)7-9-22(21)23(26)11-12-24(26)29-25(28)13-6-17-4-2-3-5-17/h8,10,16-17,21-24,27H,2-7,9,11-15H2,1H3/t21?,22?,23?,24-,26-/m0/s1
313-06-4 Process route
-

- 633-36-3
3,17β-bis-(3-cyclopentyl-propionyloxy)-estra-1,3,5(10)-triene

-

- 313-06-4
estradiol 17β-cypionate
| Conditions | Yield |
|---|---|
|
With potassium carbonate;
|
-

- 50-28-2
estradiol

-

- 313-06-4
estradiol 17β-cypionate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: pyridine
2: aqueous methanol. K2CO3
With pyridine; potassium carbonate;
|
313-06-4 Upstream products
-
633-36-3

3,17β-bis-(3-cyclopentyl-propionyloxy)-estra-1,3,5(10)-triene
-
50-28-2

estradiol
Relevant Products
-
4-Hydroxyphenethyl alcoholCAS NO.: 501-94-0
CAS:501-94-0