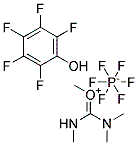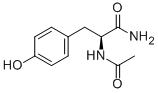
1948-71-6
- Product Name:N-Acetyl-L-tyrosinamide
- Molecular Formula:C11H14N2O3
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 1948-71-6
Molecular Formula: C11H14N2O3
Appearance: white crystalline powder
1948-71-6 Properties
- Molecular Formula:C11H14N2O3
- Molecular Weight:222.244
- Appearance/Colour:white crystalline powder
- Vapor Pressure:3.49E-14mmHg at 25°C
- Melting Point:223-225 °C(lit.)
- Refractive Index:1.576
- Boiling Point:459.53 °C at 760 mmHg
- Flash Point:231.716 °C
- PSA:92.42000
- Density:1.253 g/cm3
- LogP:1.01590
1948-71-6 Usage
Chemical Properties
WHITE CRYSTALLINE POWDER
Uses
N-Acetyl-L-tyrosine Amide is an analogue of L-tyrosine which stimulate the transport of L-Tyr, mainly by the ASC system.
InChI:InChI=1/C11H14N2O3/c1-7(14)13-10(11(12)16)6-8-2-4-9(15)5-3-8/h2-5,10,15H,6H2,1H3,(H2,12,16)(H,13,14)/t10-/m0/s1
1948-71-6 Relevant articles
Photoinduced electron transfer-promoted debenzylation of phenylalanine and tyrosine derivatives using dicyanoarene
Yamawaki, Mugen,Okita, Yoshiki,Yamamoto, Takashi,Morita, Toshio,Yoshimi, Yasuharu
, p. 7239 - 7244 (2017/11/20)
Photoinduced debenzylations of phenylalanine and tyrosine derivatives with dicyanoarenes afford glycine derivatives by the generation of radical cations. Despite the limited substrate scope, the radical cation of phenylalanine and tyrosine derivatives bearing both a carbamate (without an aromatic group) at the N-terminal and an amide at the C-terminal could promote the breaking C–C bond at the benzylic position by a photoinduced electron transfer. It is important to understand the chemical behavior of the radical cations of phenylalanine and tyrosine in enzymes involving electron transfer.
Electron Transfer Reactions of Tryptophan and Tyrosine Derivatives
Jovanovic, Slobodan V.,Harriman, Anthony,Simic, Michael G.
, p. 1935 - 1939 (2007/10/02)
Oxidation of tryptophan, tyrosine, and their derivatives by oxidizing radicals was studied by pulse radiolysis in aqueous solutions at 20 deg C.Rate constants for the oxidation of tryptophan derivatives with .N3 and Br2-. radicals vary from 8x108 to 4.8 x 109 M-1 s-1 and oxidation goes to completion; no pH dependence was observed.Oxidation rate constants for tyrosine derivatives increase upon deprotonation of the phenolic residue at higher pH.Redox potential for the indolyl and phenoxyl radicals were derived from the measured equilibrium constants by using p-methoxiphenol (E7.5 = 0.6 and E13 = 0.4 V), bisulfite (E3 = 0.84 V), and guanosine (E7 = 0.91 V) redox couples as reference systems.The redox potential of the tryptophyl radical was measured by pulse radiolysis and laser photolysis and found, by both techniques, to be E =0.64 V at pH 7.Redox potentials of tryptophan derivatives were found to be dependent on the nature of the side chain possibly due to interaction of the side chain with the nitrogen atom in the pirolle ring.Redox potentials of tyrosine derivatives were found to be independent of the nature of the side chain and higher than the redox potentials of triptophan derivatives.E = 0.85 V and E13 = 0.65 V were measured for the tyrosine/phenoxylradical redox couple at pH 7 and 13, respectively .Electron transfer from tyrosine to tryptophyl radical was found to be slow in neutral media, k = 5x105-1.3 x 106 M-1 s-1, and is suggested to proceed via multiple steps, one of which is proton transfer from tyrosine to tryptophyl radical folowed by electron transfer.
The effect of methanol on the hydrolysis of acetyl-L-tyrosinamide by
KAUFMAN,NEURATH
, p. 181 - 187 (2007/11/03)
-
1948-71-6 Process route
-

- 537-55-3
N-acetyl-L-tyrosine

-

- 1948-71-6
N-acetyl-L-tryrosinamide
| Conditions | Yield |
|---|---|
|
With dmap; ammonium chloride; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 0 - 20 ℃;
|
-

- 2440-79-1
N-acetyl-L-tyrosine methyl ester

-

- 1948-71-6
N-acetyl-L-tryrosinamide
| Conditions | Yield |
|---|---|
|
With ammonia; at -40 ℃;
|
|
|
With ammonia;
|
1948-71-6 Upstream products
-
840-97-1

N-acetyl-L-tyrosine ethyl ester
-
2440-79-1

N-acetyl-L-tyrosine methyl ester
-
100927-21-7

DL-tryptophanamide radical
-
1080-06-4

L-Tyr-OMe
1948-71-6 Downstream products
-
103383-43-3

3-{(E)-3-[4-((S)-2-Acetylamino-2-carbamoyl-ethyl)-phenoxy]-3-ethylamino-acryloyl}-benzenesulfonic acid
-
537-55-3

N-acetyl-L-tyrosine
-
503157-91-3

N-α-acetyl-O-(tert-butyl(dimethyl)silyl)-L-tyrosinamide
-
503157-93-5

N-α-(acetyl-tert-butoxycarbonyl)-N,N-di(tert-butoxycarbonyl)-L-tyrosinamide
Relevant Products
-
C.I. Pigment Blue 28
CAS:1345-16-0
-
5'-Deoxy-5-fluorocytidine
CAS:66335-38-4