Product Details;
CasNo: 629-11-8
Molecular Formula: C6H14O2
Appearance: white waxy flakes
629-11-8 Properties
- Molecular Formula:C6H14O2
- Molecular Weight:118.176
- Appearance/Colour:white waxy flakes
- Vapor Pressure:0.53 mm Hg ( 20 °C)
- Melting Point:38-42 °C(lit.)
- Refractive Index:1.457
- Boiling Point:239.7 °C at 760 mmHg
- PKA:14.87±0.10(Predicted)
- Flash Point:101.7 °C
- PSA:40.46000
- Density:0.963 g/cm3
- LogP:0.53140
629-11-8 Usage
Description
1,6-Hexanediol is a waxy hygroscopic solid compound that is white in colour. The compound is a linear diol that contains two primary hydroxyl groups that are located at the terminal. 1,6-Hexanediol’s linear hydrocarbon chain enables the compound to have enhanced hardness and flexibility of polyesters. Moreover, this property is utilized in the extending chains in polyurethanes.
Preparation
1,6-Hexanediol is produced by a propriety process that is based on BASF technology. Industrially, it is prepared by the hydrogenation of adipic acid. Conversely, in the laboratory, 1,6-Hexanediol can be synthesized by the reduction of adipic acid with lithium aluminum hydride.
Uses and Applications
Polyurethanes 1,6-Hexanediol is widely utilized in the manufacture of polyesterols such as sebacates, azelates, and adipates. These compounds are resistant to hydrolysis and have low glass transition temperature as well as high mechanical levels. 1,6-hexanediol is used as an ingredient in the preparation of a wide range of tailor-made products for numerous specialty and standard applications. In Acrylics 1,6-hexanediol is utilized as an ingredient in the manufacture of the bifunctional hexanediol diacrylate which is a monomer that is normally used in conjunction with other acrylic monomers as a reactive diluent for decorative coatings and printing inks. In Adhesives Urethanes and co-terephthalates that are based on 1,6-hexanediol provide faster better tack properties and crystallization. Due to its low glass transition property, 1,6-hexanediol offers high flexibility as well as excellent adhesive properties. Other Uses 1,6-hexanediol is incorporated into the production of other compounds used in polymeric thickeners, sizing agents, plasticizers for polyvinyl chloride, pesticides, and surfactants dyestuffs as a flexible building block.
Safety
1,6-hexanediol is a no-irritating to the skin. However, it can be irritative to the respiratory tract and mucous membrane. 1,6-hexanediol vapours or dust cause irritation to the eye. Severe eye exposure may cause conjunctivitis, iritis, and diffuse corneal opacity.
Chemical Properties
white waxy flakes
Uses
Solvent, intermediate for high polymers (nylon, polyesters), coupling agent, coil coating.
Uses
1,6-Hexanediol is used in polymer synthesis such as polyester, polyurethane and nylon. It is used as an intermediate to adhesives, acrylics and dyestuffs. Further, it is employed in gasoline refining and pharmaceutical production.
Uses
1,6-Hexanediol can be used for a variety of applications such as:a structure-directing agent for the synthesis of ZSM-5 zeolitea solvent for titanium tetraisopropoxide to form titanium oxide (TiO2) nanocrystalsa phase change material in combination with lauric acid for thermal energy storage applications
Definition
ChEBI: A diol that is hexane substituted by hydroxy groups at positions 1 and 6.
Synthesis Reference(s)
Tetrahedron Letters, 34, p. 243, 1993 DOI: 10.1016/S0040-4039(00)60557-9
Hazard
Toxic by ingestion.
Purification Methods
Fractionally crystallise it from its melt or from water. Distil it in vacuo. [Beilstein 1 IV 2556.]
629-11-8 Relevant articles
Determining Roles of Cu0 in the Chemosynthesis of Diols via Condensed Diester Hydrogenation on Cu/SiO2 Catalyst
Wang, Weichao,Wang, Hui,Zhang, Jingwei,Kong, Lingxin,Huang, Huijiang,Liu, Wei,Wang, Shengping,Ma, Xinbin,Zhao, Yujun
, p. 3849 - 3852 (2020)
Copper-based catalyst was applied in the condensed diester hydrogenation with unexpected high selectivity (~100 percent) to 1,6-hexanediol. On basis of the mass transfer analysis and kinetics results, the reaction rate of the condensed diester hydrogenation was deduced to be controlled by the activation of hydrogen on Cu0 sites, which was further demonstrated by the correlations between the catalytic activity and different copper species. Importantly, this catalysis mechanism is different with that of gas-phase diester hydrogenation, which is generally determined by the adsorption of ester on Cu+ species.
CuZn Catalysts Superior to Adkins Catalysts for Dimethyl Adipate Hydrogenolysis
Pospelova, Violetta,Aubrecht, Jaroslav,Kikhtyanin, Oleg,Pacultová, Kate?ina,Kubi?ka, David
, p. 2169 - 2178 (2019)
Industrial hydrogenolysis of esters to alcohols relies on the use of Adkins catalysts whose production and disposal is an environmental burden. This work is focused on CuZn catalysts that represent an ecological alternative to Adkins catalysts. Four CuZn catalysts with Cu/Zn atomic ratio ranging from 0.5 to 2.0 and single phase CuO and ZnO catalysts were prepared by co-precipitation and their hydrogenolysis activity was compared with a commercial Adkins catalyst. Dimethyl adipate was used to test the catalyst performance in a flow reactor at temperatures ranging from 175 to 205 °C and hydrogen pressure of 16 MPa. The increase in ZnO content was directly responsible for the reduction in copper crystallite size and increase in the catalyst specific surface area. The CuZn catalysts exhibited higher conversion than the Adkins catalyst despite their specific surface area declined during the experiments more significantly than that of the Adkins catalyst. Nonetheless, the TOF of CuZn catalysts exceeded that of the commercial Adkins catalyst.
-
Raphael,Roxburgh
, p. 3875 (1952)
-
On the selective acid-catalysed dehydration of 1,2,6-hexanetriol
Nolan, Michael R.,Sun, Geng,Shanks, Brent H.
, p. 2260 - 2266 (2014)
Selectivity results for the dehydration of 1,2,6-hexanetriol over solid acid catalysts are reported. A slate of catalysts including zeolites, amorphous silica-alumina, and niobias were tested and the selectivity towards either cyclic ethers or α,ω-dioxygenates was found to be mildly correlated with the acid strength of the fresh catalyst. In general, a ring closing dehydration reaction to a pyran was the dominant reaction pathway. Differences in the catalysts were mitigated by significant coke formation.
Synthesis of Supported RhMo and PtMo Bimetallic Catalysts by Controlled Surface Reactions
Alba-Rubio, Ana C.,Sener, Canan,Hakim, Sikander H.,Gostanian, Thomas M.,Dumesic, James A.
, p. 3881 - 3886 (2015)
We previously described a synthesis method to prepare bimetallic catalysts with narrow nanoparticle size and composition distributions by means of controlled surface reactions (CSR) between a reduced supported metal nanoparticle and an organometallic precursor of an oxophilic promoter metal. Herein, we report a comparison of such catalysts with those prepared by traditional incipient wetness impregnation. STEM/EDS analysis indicates that catalysts prepared by CSR exhibit more effective interaction of metals, thereby minimizing the undesirable formation of component-rich nanoparticles and/or monometallic domains. Reaction kinetics studies using these bimetallic catalysts reveal that optimal conversion rates in a selective CO hydrogenolysis reaction (i.e., hydrogenolysis of 2-(hydroxymethyl)tetrahydropyran to 1,6-hexanediol) could be achieved using a lower amount of the oxophilic promoter metal for the catalysts prepared by the CSR approach, as compared to their impregnated counterparts. A superior method for greater results: At the same conversion rate level, catalysts prepared by controlled surface reactions (CSR) requires smaller amount of promoter as compared to those prepared by incipient wetness impregnation (IWI). This increased performance is attributed to the uniform bimetallic composition of the catalysts prepared by CSR.
Ti3+ Tuning the Ratio of Cu+/Cu0 in the Ultrafine Cu Nanoparticles for Boosting the Hydrogenation Reaction
Zhang, Ziyang,Wang, Zhong-Li,An, Kang,Wang, Jiaming,Zhang, Siran,Song, Pengfei,Bando, Yoshio,Yamauchi, Yusuke,Liu, Yuan
, (2021)
Hydrogenation of diesters to diols is a vital process for chemical industry. The inexpensive Cu+/Cu0-based catalysts are highly active for the hydrogenation of esters, however, how to efficiently tune the ratio of Cu+/Cu0 and stabilize the Cu+ is a great challenge. In this work, it is demonstrated that doped Ti ions can tune the ratio of Cu+/Cu0 and stabilize the Cu+ by the Ti-O-Cu bonds in Ti-doped SiO2 supported Cu nanoparticle (Cu/Ti–SiO2) catalysts for the high conversion of dimethyl adipate to 1,6-hexanediol. In the synthesis of the catalysts, the Ti4+-O-Cu2+ bonds promote the reduction of Cu2+ to Cu+ by forming Ti3+-OV-Cu+ (OV: oxygen vacancy) bonds and the amount of Ti doping can tune the ratio of Cu+/Cu0. In the catalytic reaction, the O vacancy activates C=O in the ester by forming new Ti3+δ-OR-Cu1+δ bonds (OR: reactant oxygen), and Cu0 activates hydrogen. After the products are desorbed, the Ti3+δ-OR-Cu1+δ bonds return to the initial state of Ti3+-OV-Cu+ bonds. The reversible Ti-O-Cu bonds greatly improve the activity and stability of the Cu/Ti–SiO2 catalysts. When the content of Ti is controlled at 0.4?wt%, the conversion and selectivity can reach 100% and 98.8%, respectively, and remain stable for 260 h without performance degradation.
Reductive depolymerization of polyesters and polycarbonates with hydroboranes by using a lanthanum(iii) tris(amide) catalyst
Berthet, Jean-Claude,Cantat, Thibault,Kobylarski, Marie
supporting information, p. 2830 - 2833 (2022/03/09)
The homogeneous reductive depolymerization of polyesters and polycarbonates with hydroboranes is achieved with the use of an f-metal complex catalyst. These polymeric materials are transformed into their value-added alcohol equivalents. Catalysis proceeds readily, under mild conditions, with La[N(SiMe3)2]3 (1 mol%) and pinacolborane (HBpin) and shows high selectivity towards alcohols and diols, after hydrolysis.
Dinuclear copper catalyst for the oxidation/oxygenation of hydrocarbons
-
Page/Page column 23, (2021/12/02)
The subject invention provides synthetic compounds, and compound complexes having catalytic activities towards oxidation or oxygenation, and/or dehydrogenation of various substrates comprising C—H bonds. The catalysts of the subject invention comprise a d
Towards efficient Cu/ZnO catalysts for ester hydrogenolysis: The role of synthesis method
Aubrecht, Jaroslav,Kikhtyanin, Oleg,Kubi?ka, David,Pospelova, Violetta
, (2021/08/21)
Cu/ZnO catalysts represent an environmentally friendly alternative to Adkins catalysts used for ester hydrogenolysis. Cu/ZnO are mostly synthesized by co-precipitation (COP); however, other synthesis methods may help to obtain small highly dispersed Cu crystallites advantageous for catalyst activity. A comparative study on the effect of synthesis method on Cu/ZnO catalysts properties and activity is missing. Thus, we synthesized 8 wt% Cu/ZnO catalysts by five methods (COP, deposition-precipitation (DP), chemisorption-hydrolysis (CH), incipient wetness (IWI) and wet impregnation (WI)), characterized and tested them in dimethyl adipate hydrogenolysis. The CH-prepared catalyst was prone to Cu sintering, which impaired its performance. IWI led to large Cu nanoparticles, pore blocking and poor catalytic performance. COP and DP resulted in the smallest Cu nanoparticles (13?14 nm), largest Cu surface area (3.9–4.2 m2 gcat?1) and specific surface area (40?43 m2 gcat?1) reflected in their superior catalytic activity making DP a good alternative to COP to prepare active Cu/ZnO catalysts.
629-11-8 Process route
-

- 64-17-5
ethanol

-

- 141-28-6
diethyl adipate

-

- 629-11-8
1,6-hexanediol

-

- 611-10-9
2-ethoxycarbonyl-1-cyclopentanone
| Conditions | Yield |
|---|---|
|
|
-

- 627-93-0
hexanedioic acid dimethyl ester

-

- 629-11-8
1,6-hexanediol

-

- 2035-75-8
adipic anhydride
| Conditions | Yield |
|---|---|
|
With water; hydrogen; In methanol; under 37503.8 Torr;
|
89.1% 8.3% |
629-11-8 Upstream products
-
100-72-1

Tetrahydropyran-2-methanol
-
124-04-9

Adipic acid
-
629-03-8

1 ,6-dibromohexane
-
629-09-4

1,6-diiodohexane
629-11-8 Downstream products
-
101952-63-0

nicotinic acid-(6-hydroxy-hexyl ester)
-
101952-83-4

1,6-bis-nicotinoyloxy-hexane
-
2916-20-3

1,6-bis-chlorocarbonyloxy-hexane
-
13926-70-0

1,8-dioxa-cycloheptadecane-9,17-dione
Relevant Products
-
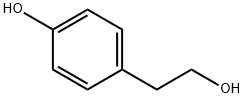
4-Hydroxyphenethyl alcoholCAS NO.: 501-94-0
CAS:501-94-0
-
Ethene,1,1'-[oxybis(2,1-ethanediyloxy)]bis-
CAS:764-99-8
-
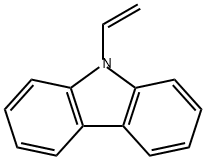
9H-Carbazole,9-ethenyl-
CAS:1484-13-5