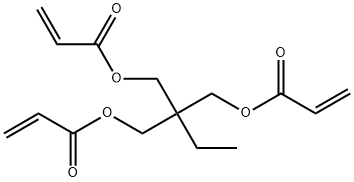
15625-89-5
- Product Name:2-Propenoicacid, 1,1'-[2-ethyl-2-[[(1-oxo-2-propen-1-yl)oxy]methyl]-1,3-propanediyl] ester
- Molecular Formula:C15H20O6
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 15625-89-5
Molecular Formula: C15H20O6
Appearance: Pale yellow to yellow transparent liquid
15625-89-5 Properties
- Molecular Formula:C15H20O6
- Molecular Weight:296.32
- Appearance/Colour:Pale yellow to yellow transparent liquid
- Vapor Pressure:<0.01 mm Hg ( 20 °C)
- Melting Point:-66 °C
- Refractive Index:n20/D 1.474(lit.)
- Boiling Point:380.9 °C at 760 mmHg
- Flash Point:165 °C
- PSA:78.90000
- Density:1.089 g/cm3
- LogP:1.57040
15625-89-5 Usage
Description
Trimethylolpropane triacrylate (TMPTA) is a multifunctional acrylie monomer. It reacts with propyleneimine to form polyfunctional aziridine. Sensitization was observed in a textile fabric printer. Patch tests were positive with the polyfunctional aziridine hardener, but were negative to TMPTA. TMPTA caused contact dermatitis in an optic-fiber manufacturing worker and was reported as a sensitizer in a floor top coat or in photopolymerizable inks.
Chemical Properties
Colorless viscid liquid
Uses
Trimethylol propane triacrylate is a triacrylate for use in UV -curable lithographic inks, varnishes, artificial nails, wood finish solder, and etch resists in the electronics industry.
Uses
Trimethylolpropane triacrylate has important properties like low volatility, resistance against weather, chemical, water and abrasion, and immediate curing property. The above mentioned properties prompted its use in the production of adhesives, plastics, sealant chemicals, paint additives, photosensitive chemicals, ink and toner. It finds application in alkyd coatings, dental polymers, compact discs, hardwood floors, concrete polymers, screen printing, elastomers, automobile headlamps, acrylics and plastic components for the medical industry.
Preparation
Synthesis of trimethylolpropane triacrylate: 2.44 kg of trimethylolpropane, 3.93 kg of acrylic acid, 0.5 kg of an acid ion exchanger (Lewatit 3333 of BAYER AG), 9 g of hydroquinone and 1.5 liters of petroleum ether (boiling range 60° to 70° C) were heated under a water separator, whilst stirring, in a 10 liter three-necked flask equipped with a stirrer, water separator and gas inlet tube. At the same time, a constant stream of air saturated with allyl alcohol was passed through the flask at a rate of 2 liters/hour. In total, 16 ml of allyl alcohol were introduced into the esterification mixture in this way. After 80 hours, the reaction mixture had an acid number of 32 and the boiling point had risen to 72° C. The petroleum ether was distilled off, remnants being removed by applying a vacuum of 0.2 mm Hg at a product temperature of 40° C. The esterification catalyst was separated off by filtration and was kept for further use. The trimethylolpropane triacrylate obtained in practically quantitative yield was pale yellowish and clear. The viscosity corresponded to a time of outflow of 20 seconds measured in a DIN cup 4 at 20° C. The product showed no change even on storage for 4 months at 60° C. When used in a UV light-curing printing ink which contained a photoinitiator, the product polymerised at high speed.Literature source US04059721
Flammability and Explosibility
Notclassified
InChI:InChI=1/C15H20O9/c16-8-15(9-17,10-18)7-14(4-1-11(19)20,5-2-12(21)22)6-3-13(23)24/h1-6,16-18H,7-10H2,(H,19,20)(H,21,22)(H,23,24)/p-3/b4-1+,5-2+,6-3+
15625-89-5 Relevant articles
Synthesis and characterization of novel ionophores of double-armed penta-crown ethers
Zhi, Bin Huang,Seung, Hyun Chang
, p. 5351 - 5355 (2005)
Unique structures of novel ionophores of double-armed penta-crown ethers were successfully synthesized. Diaza 18-crown-6 was designed as the parent crown ring. The penta-crown ethers were prepared by the reaction of trimethylolpropane triacrylate (TMPTA)
Synthesis of novel tris-crown ether structures
Huang, Zhi Bin,Chang, Seung Hyun
, p. 1703 - 1706 (2005)
Six unique tris-crown ether structures were successfully synthesized from trimethylolpropane triacrylate (TMPTA) with amino- and aza-crown ethers through Michael addition. The crown ethers contained a primary amine group such as 2-aminomethyl crown ethers and 4-aminobenzo crown ethers, others contained a secondary amine group, like 1-aza crown ethers. Georg Thieme Verlag Stuttgart.
Synthesis method of trimethylolpropane triacrylate
-
Paragraph 0021-0042, (2020/07/12)
The invention provides a synthesis method of trimethylolpropane triacrylate. The synthesis method comprises the following steps: step 1, sequentially adding trimethylolpropane, acryloyl chloride, triethylamine, hydroquinone and p-hydroxyanisole into a reaction kettle in proportion; controlling the reaction system to perform stirring reaction at the temperature of 40-60 DEG C for 2-3 hours; and step 2, after the reaction is completed, filtering the reaction solution to remove insoluble solid impurities, washing with water, and drying to obtain the product. Acryloyl chloride is used as a raw material to replace acrylic acid, and the reaction activity of acryloyl chloride is higher than that of acrylic acid so that the reaction temperature is reduced in the synthesis process, and the reactiontime is shortened; acryloyl chloride participates in the reaction, no water is generated, the use of a water-carrying agent methylbenzene or cyclohexane is avoided, the production cost is reduced, and the pollution of the organic solvent to the environment is also avoided.
Preparation method of trimethylolpropane triacrylate
-
Paragraph 0010; 0011; 0012; 0013; 0014; 0015, (2017/07/11)
Compared with the prior art, a preparation method of trimethylolpropane triacrylate has the advantages that unreacted substances in reaction products are removed or reduced through resin exchange adsorption, different from neutralization, extraction and washing technologies, no washing wastewater is produced basically in the production process, discharge of wastewater during the production is reduced, exchange resin can be recycled after adsorption saturation and utilized after being activated, and the method is a novel, energy-saving, consumption-reducing and environment-friendly technology. Secondarily, washing is not needed, product waste caused by washing is avoided, the product yield is increased, and the yield can be increased by 5% or above.
Chemoselective Transesterification of Acrylate Derivatives for Functionalized Monomer Synthesis Using a Hard Zinc Alkoxide Generation Strategy
Nakatake, Daiki,Yazaki, Ryo,Ohshima, Takashi
supporting information, p. 3696 - 3699 (2016/08/20)
A new practical method for the synthesis of functionalized acrylate derivatives with the view to prepare functional polymers was explored. Hard zinc alkoxide generation enabled the highly chemoselective transesterification of acrylate derivatives over the undesired conjugate addition, which caused polymerization. The combined use of the catalytic zinc cluster Zn4(OCOCF3)6O and 4-(dimethylamino)pyridine delivered various functionalized acrylate derivatives through the transesterification of commercially available methyl acrylate derivatives with functionalized alcohols under mild conditions.
15625-89-5 Process route
-

- 77-99-6
1,1,1-tri(hydroxymethyl)propane

-

- 79-10-7
acrylic acid

-

- 15625-89-5
2-((acryloyloxy)methyl)-2-ethylpropane-1,3-diyl diacrylate
| Conditions | Yield |
|---|---|
|
With methanesulfonic acid; hypophosphorous acid; hydroquinone; In cyclohexane; at 70 - 91 ℃; under 3750.38 Torr;
|
95% |
|
With hypophosphorous acid; 4-methoxy-phenol; copper dichloride; In cyclohexane; water; at 70 - 75 ℃;
1,1,1-tri(hydroxymethyl)propane; acrylic acid; With toluene-4-sulfonic acid; In cyclohexane; water; at 95 - 130 ℃; for 6 - 10h;
|
|
|
With methanesulfonic acid; hydroquinone; In toluene; at 120 ℃; for 5h;
|
-

- 77-99-6
1,1,1-tri(hydroxymethyl)propane

-

- 814-68-6,25189-84-8
acryloyl chloride

-

- 15625-89-5
2-((acryloyloxy)methyl)-2-ethylpropane-1,3-diyl diacrylate
| Conditions | Yield |
|---|---|
|
With 4-methoxy-phenol; triethylamine; hydroquinone; at 60 ℃; for 2h; Temperature;
|
98.38% |
|
In pyridine; benzene; at 50 ℃; for 30h;
|
81% |
|
With pyridine; In benzene; at 50 ℃; for 30h;
|
81% |
15625-89-5 Upstream products
-
77-99-6

1,1,1-tri(hydroxymethyl)propane
-
814-68-6

acryloyl chloride
-
79-10-7

acrylic acid
-
37275-47-1

trimethylolpropane diacrylate
15625-89-5 Downstream products
-
107-15-3

ethylenediamine
-
1260149-11-8

C50H70N4O13
-
1030374-82-3

C28H30O7S
-
1591653-39-2

trimethylolpropane tris[3-(bis(2,4,6-trimethylbenzoyl)phosphoryl)propanoate]
Relevant Products
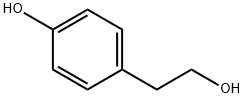
-
4-Hydroxyphenethyl alcoholCAS NO.: 501-94-0
CAS:501-94-0
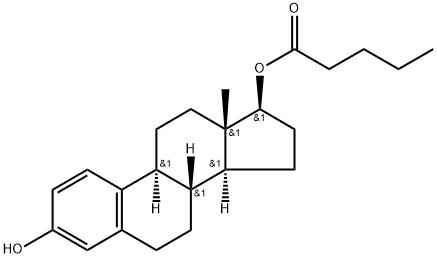
-
Estradiol valerate
CAS:979-32-8
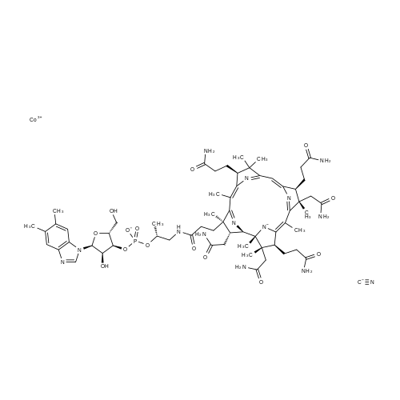
-
Cyanocobalamin
CAS:68-19-9