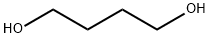
Product Details;
CasNo: 110-63-4
Molecular Formula: C4H10O2
Appearance: viscous colourless liquid
1,4-Butanediol
- Molecular Formula:C4H10O2
- Molecular Weight:90.1222
- Appearance/Colour:viscous colourless liquid
- Vapor Pressure:0.015mmHg at 25°C
- Melting Point:20 °C
- Refractive Index:n20/D 1.445(lit.)
- Boiling Point:227.999 °C at 760 mmHg
- PKA:14.73±0.10(Predicted)
- Flash Point:105.909 °C
- PSA:40.46000
- Density:1.006 g/cm3
- LogP:-0.24880
1,4-Butanediol(Cas 110-63-4) Usage
|
|
|
|
Sources and Categories |
1,4-Butanediol (1,4-BDO) is a tetracarbon diol and an important commodity chemical. It is synthesized through various chemical processes and can also be produced bio-based approaches. It serves as a platform chemical for the synthesis of various chemicals and materials, including polyesters, polyurethanes, tetrahydrofuran, and γ-butyrolactone. |
|
Market Data |
In 2015, the total market size for chemicals and polyesters manufactured using 1,4-BDO as a raw material was valued at over USD 6.19 billion. It is a significant commodity chemical with a growing market. |
|
Mechanism of Action |
1,4-Butanediol serves as a precursor in various chemical reactions for the synthesis of polyurethanes, polyesters, and other chemicals. |
|
Industrial Applications |
Used in the synthesis of polyurethane elastomers, polyesters (PBT, PBAT), spandex fibers, and special-purpose coatings. Also employed as organic solvents and raw materials for organic synthesis. |
|
Production Methods |
Chemical catalysis processes such as hydrogenation of maleic anhydride, isomerization of propylene oxide, acetoxylation of butadiene, and reaction between formaldehyde and acetylene are the main industrial methods for 1,4-BDO production. |
|
General Description |
1,4-Butanediol is a versatile chemical intermediate used in various synthetic applications, including the production of macrolides, hydrogels, and marine natural products. It serves as a precursor or building block in reactions such as ring-closing metathesis, esterification, and reductive cyclization, contributing to the synthesis of complex molecules like brevisamide, stagonolide C, and modiolide A. Additionally, it is employed in the fabrication of biocompatible hydrogels, where its incorporation enhances material properties for biomedical uses. Its role in asymmetric synthesis further highlights its utility in producing enantiomerically enriched compounds. |
InChI:InChI=1/C4H10O2/c5-3-1-2-4-6/h5-6H,1-4H2
110-63-4 Relevant articles
Effect of boron content on 1,4-butanediol production by hydrogenation of succinic acid over Re-Ru/BMC (boron-modified mesoporous carbon) catalysts
Kang, Ki Hyuk,Han, Seung Ju,Lee, Jong Won,Kim, Tae Hyeop,Song, In Kyu
, p. 206 - 213 (2016)
A series of Re-Ru bimetallic catalysts s...
Hydrogenation of γ-Butyrolactone to 1,4-Butanediol over CuCo/TiO2 Bimetallic Catalysts
Huang, Zhiwei,Barnett, Kevin J.,Chada, Joseph P.,Brentzel, Zachary J.,Xu, Zhuoran,Dumesic, James A.,Huber, George W.
, p. 8429 - 8440 (2017)
Titania-supported monometallic and bimet...
Extremely facile and selective nickel-catalyzed allyl ether cleavage
Taniguchi, Takahiko,Ogasawara, Kunio
, p. 1136 - 1137 (1998)
Child's play! Allyl ethers as protecting...
Thermodynamic equilibria between polyalcohols and cyclic ethers in high-temperature liquid water
Yamaguchi, Aritomo,Hiyoshi, Norihito,Sato, Osamu,Bando, Kyoko K.,Masuda, Yoshio,Shirai, Masayuki
, p. 2666 - 2668 (2009)
Thermodynamic equilibrium constants betw...
-
Enz,W.
, p. 206 - 212 (1961)
-
A temperature-controlled reversible ionic liquid - Water two phase - Single phase protocol for hydrogenation catalysis
Dyson,Ellis,Welton
, p. 705 - 708 (2001)
An ionic liquid - water system that unde...
Comparison of Carbon-13 Nuclear Magnetic Resonance Methods for the Analysis of Multiple Partially Deuteriated Products from Catalytic Reactions: Heptan-1-ol and 2-Methylpropanol
MacDougall, Joanna K.,Simpson, Michael C.,Cole-Hamilton, David J.
, p. 3061 - 3066 (1994)
Products from the hydrocarbonylation of ...
A Study of the Catalytic Deuteration of 1,4-Butynediol
Chickos, James S.,Uang, Jack Y.-J.,Keiderling, Tim A.
, p. 2594 - 2596 (1991)
-
High chemo and regioselective formation of alcohols from the hydrocarbonylation of alkenes using cooperative ligand effects
Boogaerts, Ine T.I. F.,White, Daniel F. S.,Cole-Hamilton, David J.
, p. 2194 - 2196 (2010)
The hydrocarbonylation of alkenes, inclu...
Continuous hydrogenation of 2-butyne-1,4-diol to 2-butene- and butane-1,4-diols
Rode,Tayade,Nadgeri,Jaganathan,Chaudhari
, p. 278 - 284 (2006)
Continuous catalytic hydrogenation of 2-...
A new carboxylesterase from Brevibacterium linens IFO 12171 responsible for the conversion of 1,4-butanediol diacrylate to 4-hydroxybutyl acrylate: Purification, characterization, gene cloning, and gene expression in Escherichia coli
Sakai, Yasuyoshi,Ishikawa, Junko,Fukasaka, Shunji,Yurimoto, Hiroya,Mitsui, Ryoji,Yanase, Hideshi,Kato, Nobuo
, p. 688 - 697 (1999)
A carboxylesterase that is responsible f...
Effect of Ethers on Reactions of Butylcoppers with α,β-Unsaturated Ketones in Toluene
Kingsbury, Celia L.,Smith, Robin A. J.
, p. 7637 - 7643 (1997)
Mixtures of butyllithium and copper iodi...
A Novel and Unusual Reaction of Enol Ethers with Benzyltriethylammonium Borohydride and Chlorotrimethylsilane
Baskaran, S.,Chidambaram, N.,Narasimhan, N.,Chandrasekaran, S.
, p. 6371 - 6374 (1992)
Benzyltriethylammonium borohydride-chlor...
A Schiff Base Modified Pd Catalyst for Selective Hydrogenation of 2-Butyne-1,4-diol to 2-Butene-1,4-diol
Li, Haomeng,Wang, Xinkui,Chen, Xiao,Li, Chuang,Wang, Min,Yi, Yanhui,Ji, Min,Wang, Huilong,Shao, Zhengfeng,Liang, Changhai
, p. 2150 - 2157 (2020)
Abstract: Schiff-base modified Pd nanopa...
Bimetallic Synergy Effects of Phyllosilicate-Derived NiCu@SiO2 Catalysts for 1,4-Butynediol Direct Hydrogenation to 1,4-Butanediol
Wang, Changzhen,Tian, Yani,Wu, Ruifang,Li, Haitao,Yao, Benzhen,Zhao, Yongxiang,Xiao, Tiancun
, p. 4777 - 4787 (2019)
Hydrogenation of 1,4-butynediol (BYD) to...
Quantification of Interfacial pH Variation at Molecular Length Scales Using a Concurrent Non-Faradaic Reaction
Ryu, Jaeyune,Wuttig, Anna,Surendranath, Yogesh
, p. 9300 - 9304 (2018)
We quantified changes in interfacial pH ...
Modelling proposed intermediates in the hydrocarbonylation of alkenes catalysed by rhodium complexes of PBui3 and PPr i3
Cheliatsidou, Paraskevi,White, Daniel F. S.,Slawin, Alexandra M. Z.,Cole-Hamilton, David J.
, p. 2389 - 2394 (2008)
In ethanol, hydrocarbonylation reactions...
DIRECT SYNTHESIS OF 1,4-BUTANEDIOL FROM ALLYL ALCOHOL USING CARBON MONOXIDE AND WATER IN THE PRESENCE OF Rh6(CO)16-PROPANEDIAMINE SYSTEM
Kaneda, Kiyotomi,Imanaka, Toshinobu,Teranishi, Shiichiro
, p. 1465 - 1466 (1983)
Rh6(CO)16-propanediamine system catalyze...
An unusual reaction of cyclic enol ethers with titanium(III) tetrahydroborate
Ravikumar,Chandrasekaran, Srinivasan
, p. 2973 - 2978 (1997)
Titanium(III) Tetrahydroborate formed in...
Alkaline hydrolysis of oligomers of tartrate esters: Effect of a neighboring carboxyl on the reactivity of ester groups
Maniar,Kalonia,Simonelli
, p. 705 - 709 (1992)
The importance and the effect of neighbo...
New environmentally friendly catalysts containing Pd-interstitial carbon made from Pd-glucose precursors for ultraselective hydrogenations in the liquid phase
Chan, Chun Wong Aaron,Xie, Yaling,Cailuo, Nick,Yu, Kai Man Kerry,Cookson, James,Bishop, Peter,Tsang, Shik Chi
, p. 7971 - 7973 (2011)
We report a novel preparation of a Pd na...
Zirconium(IV) chloride catalyzed new and efficient protocol for the selective cleavage of p-methoxybenzyl ethers
Madhava Sharma, Gangavaram V.,Reddy, Ch. Govardhan,Krishna, Palakodety Radha
, p. 4574 - 4575 (2003)
A highly selective and efficient method ...
Efficient Pd@MIL-101(Cr) hetero-catalysts for 2-butyne-1,4-diol hydrogenation exhibiting high selectivity
Yin, Dongdong,Li, Chuang,Ren, Hangxing,Shekhah, Osama,Liu, Jinxuan,Liang, Changhai
, p. 1626 - 1633 (2017)
Pd@MIL-101(Cr) hetero-catalysts have bee...
Aqueous phase hydrogenation of acetic acid to ethanol over Ir-MoO x/SiO2 catalyst
Wang, Zhiqiang,Li, Guangyi,Liu, Xiaoyan,Huang, Yanqiang,Wang, Aiqin,Chu, Wei,Wang, Xiaodong,Li, Ning
, p. 38 - 41 (2014)
Mo modified Ir/SiO2 (Ir-MoOx/SiO2) was f...
Biosynthesis of 1,4-butanediol from erythritol using whole-cell catalysis
Dai, Lu,Tai, Cui,Shen, Yaling,Guo, Yali,Tao, Fei
, p. 1 - 5 (2018)
1,4-Butanediol (BDO) biosynthesis from r...
Raney Ni-Si catalysts for selective hydrogenation of highly concentrated 2-butyne-1,4-diol to 2-butene-1,4-diol
Chen, Xiao,Zhang, Mingming,Yang, Kaixuan,Williams, Christopher T.,Liang, Changhai
, p. 1118 - 1126 (2014)
Raney Ni-Si catalysts were synthesized b...
Kinetics and mechanism of tetrahydrofuran synthesis via 1,4-butanediol dehydration in high-temperature water
Hunter, Shawn E.,Ehrenberger, Carolyn E.,Savage, Phillip E.
, p. 6229 - 6239 (2006)
We conducted an experimental investigati...
Highly dispersed palladium nanoclusters incorporated in amino-functionalized silica spheres for the selective hydrogenation of succinic acid to γ-butyrolactone
You, Chenjia,Zhang, Chi,Chen, Lifang,Qi, Zhiwen
, p. 653 - 660 (2015)
Highly dispersed palladium nanoclusters ...
Liquid-phase catalytic hydrogenation of 2-butyne-1,4-diol to 1,4-butanediol at atmospheric pressure on suspended catalysts
Pyatnitsyna,El'Chaninov
, p. 394 - 397 (2013)
The optimum parameters of hydrogenation ...
Analysis of drugs by pyrolysis. I. Selected ion monitoring combined with a pyrolysis method for the determination of carpronium chloride in biological samples.
Ohya,Sano
, p. 241 - 247 (1977)
In order to establish an analytical meth...
Hot Water Induces an Acid-Catalyzed Reaction in Its Undissociated Form
Nagai, Yasuharu,Matubayasi, Nobuyuki,Nakahara, Masaru
, p. 691 - 697 (2004)
Hot water in its undissociated form has ...
Selective Hydrogenation of Cyclic Ester to α,ω-Diol Catalyzed by Cationic Ruthenium Complexes with Trialkylphosphine Ligands
Hara, Yoshinori,Inagaki, Hiroko,Nishimura, Sugio,Wada, Keisuke
, p. 1983 - 1986 (1992)
Cyclic esters like γ-butyrolactone were ...
Pt nanoparticles over TiO2-ZrO2 mixed oxide as multifunctional catalysts for an integrated conversion of furfural to 1,4-butanediol
Li, Fengbo,Lu, Tao,Chen, Bingfeng,Huang, Zhijun,Yuan, Guoqing
, p. 252 - 258 (2014)
1,4-butanediol (BDO) is an important com...
Tracking Electrical Fields at the Pt/H2O Interface during Hydrogen Catalysis
Ryu, Jaeyune,Surendranath, Yogesh
, p. AR (2019)
We quantify changes in the magnitude of ...
First example of borirane synthesis by α-olefins reaction with BCl3·SMe2 Catalyzed with (η5-C5H5)2TiCl2
Khusainova,Khafizova,Tyumkina,Dzhemilev
, p. 1517 - 1523 (2015)
New method was developed of the synthesi...
Converging conversion - using promiscuous biocatalysts for the cell-free synthesis of chemicals from heterogeneous biomass
Pick, André,Sieber, Volker,Sutiono, Samuel
, p. 3656 - 3663 (2021)
Production of chemicals from lignocellul...
Reductive cleavage of tert-butyldimethylsilyl ether via intramolecular transfer of hydride
Saravanan, Parthasarathy,Gupta, Suparna,DattaGupta, Arpita,Gupta, Sonia,Singh, Vinod K.
, p. 2695 - 2699 (1997)
The cleavage of α-hydroxy tert-butyldime...
Catalytic hydrogenation of 2-butyne-1,4-diol to 2-butene-1,4-diol at atmospheric pressure in the liquid phase
Pyatnitsyna,El'chaninov,Savost'yanov
, p. 89 - 92 (2006)
Selective hydrogenation of 2-butyne-1,4-...
A synthesis method of diol
-
Paragraph 0043-0048, (2022/01/12)
The present invention belongs to the fie...
Method for producing a shaped catalyst body
-
Page/Page column 29-30, (2021/11/19)
Provided herein is a novel process for p...
Hydroboration Reaction and Mechanism of Carboxylic Acids using NaNH2(BH3)2, a Hydroboration Reagent with Reducing Capability between NaBH4and LiAlH4
Wang, Jin,Ju, Ming-Yue,Wang, Xinghua,Ma, Yan-Na,Wei, Donghui,Chen, Xuenian
, p. 5305 - 5316 (2021/04/12)
Hydroboration reactions of carboxylic ac...
110-63-4 Process route
-
-
C36H60O30*C4H10O2

-
- 110-63-4
Butane-1,4-diol

-
- 10016-20-3
alpha cyclodextrin
| Conditions | Yield |
|---|---|
|
In water; at 25 ℃; Equilibrium constant;
|
-
- 624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
- 109-99-9,24979-97-3,77392-70-2
tetrahydrofuran

-
- 13436-45-8
2-methoxytetrahydrofuran

-
- 96-48-0
4-butanolide

-
- 71-23-8
propan-1-ol

-
-
1-methoxy-1,4-butanediol

-
- 64001-06-5
2-(4'-hydroxybutoxy)-tetrahydrofuran

-
-
4-hydroxy-butanoic acid 4-hydroxybutyl ester

-
- 110-63-4
Butane-1,4-diol

-
- 25714-71-0
4-hydroxybutyraldehyde

-
- 925-57-5
methyl 4-hydroxybutanoate

-
- 71-36-3
butan-1-ol
| Conditions | Yield |
|---|---|
|
With hydrogen; at 190 ℃; under 46504.7 Torr; Gas phase;
|
79.1% 10.4% 5.3% |
110-63-4 Upstream products
-
1708-29-8
2,5-dihydrofuran
-
108-31-6
maleic anhydride
-
110-52-1
1,4-dibromo-butane
-
110-65-6
1,4-dihydroxybut-2-yne
110-63-4 Downstream products
-
13179-96-9
1,4-dimethoxybutane
-
5835-79-0
4-(1-hydroxypropyl)morpholine
-
53161-64-1
4,4'-butane-1,4-diyl-bis-morpholine
-
7191-20-0
2-phenyl-1,3,2-dioxaphosphepin-2-oxide
Relevant Products
-
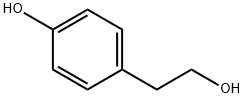
4-Hydroxyphenethyl alcoholCAS NO.: 501-94-0
CAS:501-94-0
-
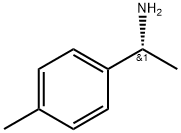
(R)-(+)-1-(4-Methylphenyl)ethylamine
CAS:4187-38-6
-
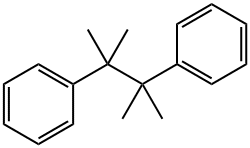
Benzene,1,1'-(1,1,2,2-tetramethyl-1,2-ethanediyl)bis-
CAS:1889-67-4