产品详情;
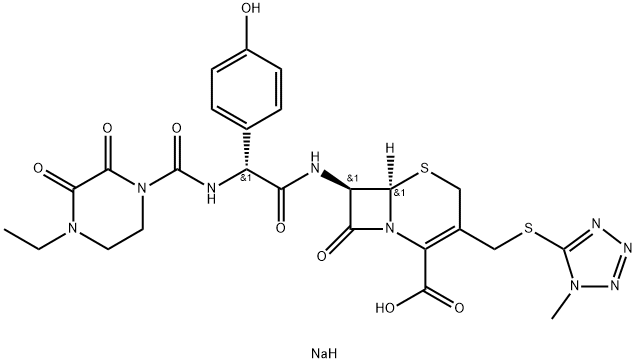
CasNo: 62893-20-3
分子式: C25H28N9NaO8S2
外观: colourless viscous liquid
交货时间: In stock
生产能力: 10000|Metric Ton|Month
技术指标: 99%
| Cefoperazone sodium Chemical Properties |
| Melting point | 200-202°C |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: 50 mg/mL, clear, faintly yellow |
| form | powder |
| Water Solubility | Soluble in water. Slightly soluble in alcohol. |
| Merck | 13,1943 |
| BRN | 4902135 |
| InChIKey | NCFTXMQPRQZFMZ-WERGMSTESA-M |
| CAS DataBase Reference | 62893-20-3(CAS DataBase Reference) |
| Safety Information |
| Hazard Codes | Xn |
| Risk Statements | 42/43 |
| Safety Statements | 22-36/37 |
| WGK Germany | 2 |
| RTECS | XI0374000 |
| HS Code | 29419000 |
| MSDS Information |
| Cefoperazone sodium Usage And Synthesis |
| Chemical Properties | Faint beige powder |
| Originator | Cefobid,Pfizer,W. Germany,1981 |
| Uses | For studying the expression, binding, and inhibition of penicillin-binding proteins.Cefoperazone sodium salt is used in the study of drug-protein binding, expression and inhibition of penicillin-binding proteins during cell wall synthesis. It is as an antibacterial and antimicrobial agent useful for prevention of postoperative infection. It is used as an inhibitor of inactivation of alfa-antitrypsin. |
| Uses | Broad spectrum third generation cephalosporin antibiotic. An antibacterial. |
| Uses | antidepressant, serotonin reuptake inhibitor, 5HT1A agonist |
| Manufacturing Process | To a suspension of 3.0 g of 7-[D-(-)-α-amino-p-hydroxyphenylacetamido]-3- [5-(1-methyl-1,2,3,4-tetrazolyl)thiomethyl]- 3-cephem-4-carboxylic acid in 29 ml of water was added 0.95 g of anhydrous potassium carbonate. After the solution was formed, 15 ml of ethyl acetate was added to the solution, and 1.35 g of 4-ethyl-2,3-dioxo-1-piperazinocarbonyl chloride was added to the resulting solution at 0°C to 5°C over a period of 15 minutes, and then the mixture was reacted at 0°C to 5°C for 30 minutes. After the reaction, an aqueous layer was separated off, 40 ml of ethyl acetate and 10 ml of acetone were added to the aqueous layer, and then the resulting solution was adjusted to a pH of 2.0 by addition of dilute hydrochloric acid. Thereafter, an organic layer was separated off, the organic layer was washed two times with 10 ml of water, dried over anhydrous magnesium sulfate, and the solvent was removed by distillation under reduced pressure. The residue was dissolved in 10 ml of acetone, and 60 ml of 2-propanol was added to the solution to deposit crystals. The deposited crystals were collected by filtration, washed with 2- propanol, and then dried to obtain 3.27 g of 7-[D-(-)-α-(4-ethyl-2,3-dioxo)-1- piperazinocarbonylamino)-p-hydroxyphenylacetamido]-3-[5-(1-methyl- 1,2,3,4-tetrazolyl)thiomethyl]- 3-cephem-4-carboxylicacid, yield 80.7%. The product forms crystals, MP 188°C to 190°C (with decomposition). |
| Brand name | Cefobid (Pfizer). |
| Therapeutic Function | Antibiotic |
| Clinical Use | Cefoperazone sodium is a third-generation, antipseudomonalcephalosporin that resembles piperacillinchemically and microbiologically. It is active against manystrains of P. aeruginosa, indole-positive Proteus spp.,Enterobacter spp., and S. marcescens that are resistant tocefamandole. It is less active than cephalothin againstGram-positive bacteria and less active than cefamandoleagainst most of the Enterobacteriaceae. Like piperacillin,cefoperazone is hydrolyzed by many of the β-lactamasesthat hydrolyze penicillins. Unlike piperacillin, however, itis resistant to some (but not all) of the β-lactamases thathydrolyze cephalosporins.Cefoperazone is excreted primarily in the bile. Hepaticdysfunction can affect its clearance from the body. |
| Veterinary Drugs and Treatments | Cefoperazone is used to treat serious infections, particularly susceptible Enterobacteriaceae not susceptible to other less expensive agents or when aminoglycosides are not indicated (due to their potential toxicity). |
相关产品
-
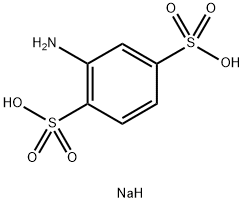
2-氨基-1,4-苯二磺酸一钠
CAS:24605-36-5
-
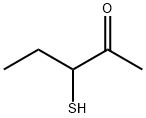
3-硫基-2-戊酮
CAS:67633-97-0
-
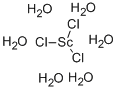
六水氯化钪
CAS:20662-14-0