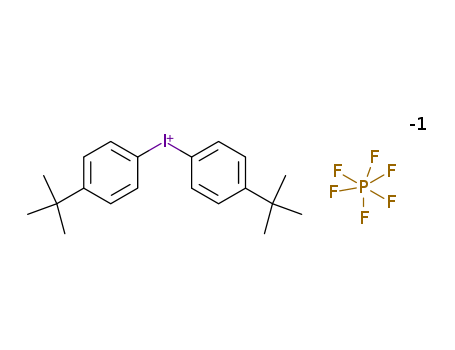
61358-25-6
- Product Name:Bis(4-tert-butylphenyl)iodonium hexafluorophosphate
- Molecular Formula:C20H26F6IP
- Purity:99%
- Molecular Weight:
Product Details;
CasNo: 61358-25-6
Molecular Formula: C20H26F6IP
Henan Wentao Chemical Product Co., Ltd. is a quality supplier and manufacturer of Bis(4-tert-butylphenyl)iodonium hexafluorophosphate . You can buy high purity, low price Bis(4-tert-butylphenyl)iodonium hexafluorophosphate 61358-25-6 here. Contact us.
High Purity Bis(4-t-butyl phenyl)iodonium hexafluorophosphate 61358-25-6 Export
- Molecular Formula:C20H26F6IP
- Molecular Weight:538.295
- Melting Point:174 °C
- PSA:13.59000
- LogP:5.79240
61358-25-6 Usage
Bis(4-(tert-butyl)phenyl)iodonium hexafluorophosphate(V) is an organic catalyst used for the photoinduced thermal polymerization reactions.
InChI:InChI=1/C20H26I.F6P/c1-19(2,3)15-7-11-17(12-8-15)21-18-13-9-16(10-14-18)20(4,5)6;1-7(2,3,4,5)6/h7-14H,1-6H3;/q+1;-1
61358-25-6 Relevant articles
Challenges and limits of upconversion nanoparticles for cationic photopolymerization with UV initiators excited at 980 nm
Paul Hermes,a Andrea Hermsen,a Martin Jäger, a Jochen S. Gutmann, b Veronika Strehmel a and Bernd Strehmel *a
Polymer Chemistry, Issue 34, 2022
A dual photo-initiating system comprising (2-i-propyl)thioxanthone (ITX) and bis(4-t-butyl)phenyl iodonium hexafluorophosphate (IS-PF6) can generate the conjugate acid via …
Bisphosphonic Acid-Functionalized Water-Soluble Photoinitiators
Tugce Nur Eren, Jacques Lalevée, Duygu Avci
Macromolecular Chemistry and Physics, Volume220, Issue19 October 2019 1900268
Photopolymerization results demonstrate that they can successfully initiate the photopolymerization of poly(ethylene glycol) diacrylate (Mn = 250 D) in the presence of bis-(4-tert-butylphenyl)-iodonium hexafluorophosphate (Iod).
61358-25-6 Process route
-

- 253185-03-4,253185-04-5
tert-butylbenzene

-

- 61358-25-6
bis(4-tert-butylphenyl)iodonium hexafluorophosphate
| Conditions | Yield |
|---|---|
|
tert-butylbenzene; With iodine; toluene-4-sulfonic acid; 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 40 ℃; for 0.25h;
With trifluorormethanesulfonic acid; In dichloromethane; at 0 - 20 ℃; for 12h;
With sodium hexaflorophosphate; In dichloromethane; water; at 20 ℃; for 12h;
|
-

- 370-69-4
tris(2,2,2-trifluoroethyl)phosphite

-

- 61358-25-6
bis(4-tert-butylphenyl)iodonium hexafluorophosphate

-

- 1825-33-8
phenylazo-i-butyric acid nitrile

-

- 358-63-4
tris(2,2,2-trifluoroethyl) phosphate

-

- 35779-04-5
1-tert-butyl-4-iodobenzene

-

- 1428647-08-8
bis(2,2,2-trifluoroethyl) tert-butylphenylphosphonate

-

- 172422-37-6
Phenyl-phosphonic acid bis-(2,2,2-trifluoro-ethyl) ester
| Conditions | Yield |
|---|---|
|
With cyclohexane-1,2-epoxide; In neat (no solvent); at 34 ℃; for 4.16667h; UV-irradiation;
|
15 %Spectr. 10 %Spectr. 14 %Spectr. 20 %Spectr. |
|
With cyclohexane-1,2-epoxide; In [D3]acetonitrile; at 34 ℃; for 1h; UV-irradiation;
|
15 %Spectr. 27 %Spectr. 14 %Spectr. 33 %Spectr. |
|
With cyclohexane-1,2-epoxide; In [D3]acetonitrile; at 34 ℃; for 4.5h; UV-irradiation;
|
32 %Spectr. 62 %Spectr. 44 %Spectr. 85 %Spectr. |
61358-25-6 Upstream products
-
253185-03-4

tert-butylbenzene
61358-25-6 Downstream products
-
462-06-6

fluorobenzene
-
591-50-4

iodobenzene
-
22904-43-4

4-tert-Butyl-phenylradikal
-
100-66-3

methoxybenzene
Relevant Products
-
1H,1H,2H,2H-Perfluorooctyltrimethoxysilane
CAS:85857-16-5
-
Progesterone
CAS:57-83-0
-
Antioxidant 5057
CAS:68411-46-1